Fluorescence Spectroscopy and Chemometric Modeling for Bioprocess Monitoring
Abstract
:1. Introduction
| Organism | Type | Cultivation | Fluorescence | Reference |
|---|---|---|---|---|
| Escherichia coli | Bacteria | Batch, Fed-Batch Continuous | 2D-Fluorescence, NAD(P)H fluorescence | [9,27,32,33,34,35,36,37,38,39,40] |
| Wautersia eutropha | Batch | NAD(P)H fluorescence | [41] | |
| Bacillus polymyxa | Batch | 2D-Fluorescence | [42] | |
| Klebsiella pneumonia | Batch | 2D-Fluorescence | [43] | |
| Aspergillus oryzae | Batch, Fed-Batch | 2D-Fluorescence | [44] | |
| Alcaligenes eutrophus | Fed-Batch | 2D-Fluorescence | [25] | |
| Aspergillus niger | Fed-Batch | 2D-Fluorescence | [45] | |
| Pseudomonas aeruginosa | Batch | 2D-Fluorescence | [38,46] | |
| Azohydromonas australica | Batch | NAD(P)H fluorescence | [20] | |
| Bacillus | Fed-Batch | 2D-Fluorescence | [47] | |
| Streptomyces coelicolor | Fed-Batch, Continuous | 2D-Fluorescence | [28,29] | |
| Pichia pastoris | Fungi | Batch | 2D-Fluorescence | [48,49,50,51] |
| Saccharomyces cerevisiae | Batch, Fed-Batch | 2D-Fluorescence | [10,11,27,37,52,53,54,55] | |
| Claviceps purpurea Hansenula polymorpha | Batch Batch | 2D-Fluorescence NAD(P)H fluorescence | [8,40] | |
| NSO Cells | Mammalian | Batch | 2D-Fluorescence | [50] |
| Baby Hamster Kidney Cells | Batch, Fed-Batch | 2D-Fluorescence | [56] | |
| Chinese Hamster Ovar Cells | Batch, Fed-Batch | 2D-Fluorescence | [30,57,58] | |
| Azadirachta indica | Plant | Batch | NAD(P)H fluorescence | [59] |
| Eschscholtzia California | Batch | 2D-Fluorescence | [60] | |
| Catharantuhus roseus | Batch | 2D-Fluorescence | [60] |
2. Fluorescence Spectroscopy
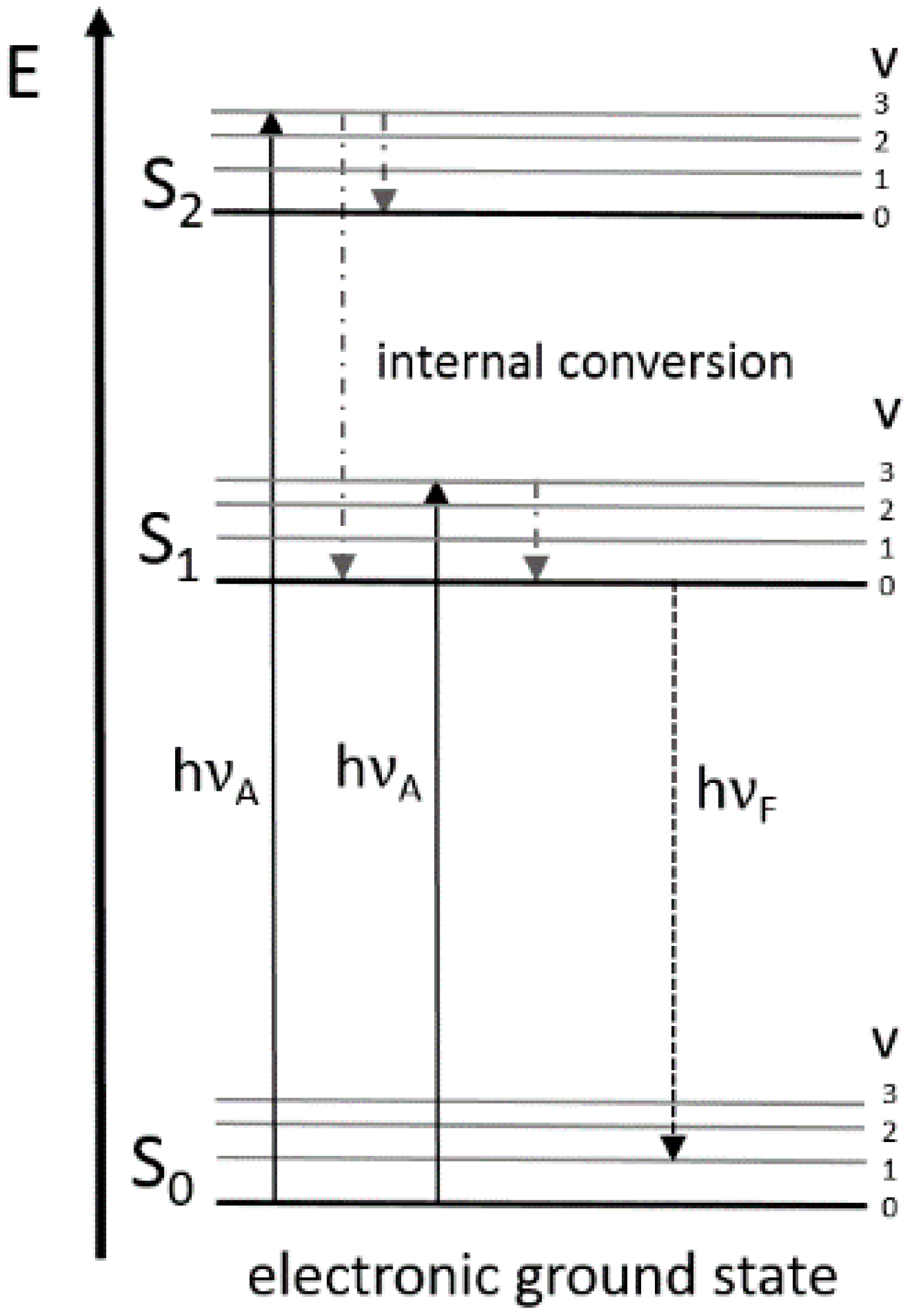
2.1. Principles and Fluorophores

| Fluorophore | Max Excitation Wavelength (nm) | Max Emission Wavelength (nm) | Reference | |
|---|---|---|---|---|
| GFP | fluorescence proteins | 400, 470 | 505, 540 | [62] |
| EYFP | 514 | 527 | [62] | |
| mCherry | 587 | 610 | [65] | |
| Tryptophan | amino acids | 280, 290 | 350 | [63,66] |
| Tyrosine | 275/278 | 280, 300/330–350 | [63,66,67] | |
| Phenylalanine | 260 | 280, 282 | [63,68] | |
| FAD, Flavins | co-enzymes | 450 | 535 | [63] |
| NADH | 290, 351 | 440, 460 | [63] | |
| NAD(P)H | 336 | 464 | [63] | |
| Pyrodoxin | vitamins | 332, 340 | 400 | [63] |
| Vitamin A | 327 | 510 | [63] | |
| Riboflavin | 365 | 520 | [66] |
2.2. Fluorescence Spectrometer
| Type | Wavelength Selector | Wavelength | Resolution | Reference |
|---|---|---|---|---|
| BioView® | Filter | Excitation: 260–560 nm Emission: 300–600 nm | 20 nm | [8,9,10,11,28,29,32,33,42,43,44,47,48,50,51,52,53,57,60,69] |
| FLUOstar® | Filter | NADH Signal | - | [34,39,40] |
| Hitachi F4500 | Grating | Excitation 200–890 nm Emission 200–900 nm | 10 nm | [11,27,35,36,37,45,46] |
| Perkin Elmer LS 50 B /55 | Grating | Excitation: 200–800 nm Emission: 200–650/900 nm | 1 nm | [26,49,56] |
| Varian Cary Eclipse | Grating | up to 900 nm | 1.5 nm | [25,30] |
| Varian VIPL 3120 | Filter | NADH Signal | - | [41,59] |
| Ingold Type Fluorosensor | Filter | Excitation: 360 nm Emission: 450 nm | - | [20] |
| USB2000 spectrometer | Grating | 200-1100 nm | 10 nm | [55] |
| FL3095 | Grating | Excitation: 260–680 nm Emission: 320–950 nm | - | [54] |

3. Extracting Information Out of Fluorescence Spectra
3.1. Preprocessing
3.2. Data Reduction, Decomposition and Wavelength Selection
| Method | Application | Software | Reference |
|---|---|---|---|
| PCA | Data evaluation, Data reduction | Unscrambler®, MATLAB® | [10,25,26,29,33,42,56,69] |
| SWR | Wavelength selection | MATLAB® | [53,55] |
| MROBPCA | Data quality and outlier detection | MATLAB® | [58] |
| MCR-ALS | Data decomposition | Unscrambler®, MATLAB® | [30,54] |
| SIMPLISMA | Data decomposition | Unscrambler®, MATLAB® | [54] |
| SOM | Data reduction, Classify spectra | MATLAB® ViscoverySOMine | [9,27,35,36,37] |
| PARAFAC | Data decomposition, Data evaluation/selection | MATLAB® | [10,29,47,48] |
| GA | Wavelength selection | MATLAB® | [28,29,53] |
| ES | Wavelength selection | MATLAB® | [53] |
| iPLS | Wavelength selection | MATLAB® | [28] |
| PV | Wavelength selection | MATLAB® | [28] |
| ACO | Wavelength selection | MATLAB® | [30] |
| CARS | Wavelength selection | MATLAB® | [30] |
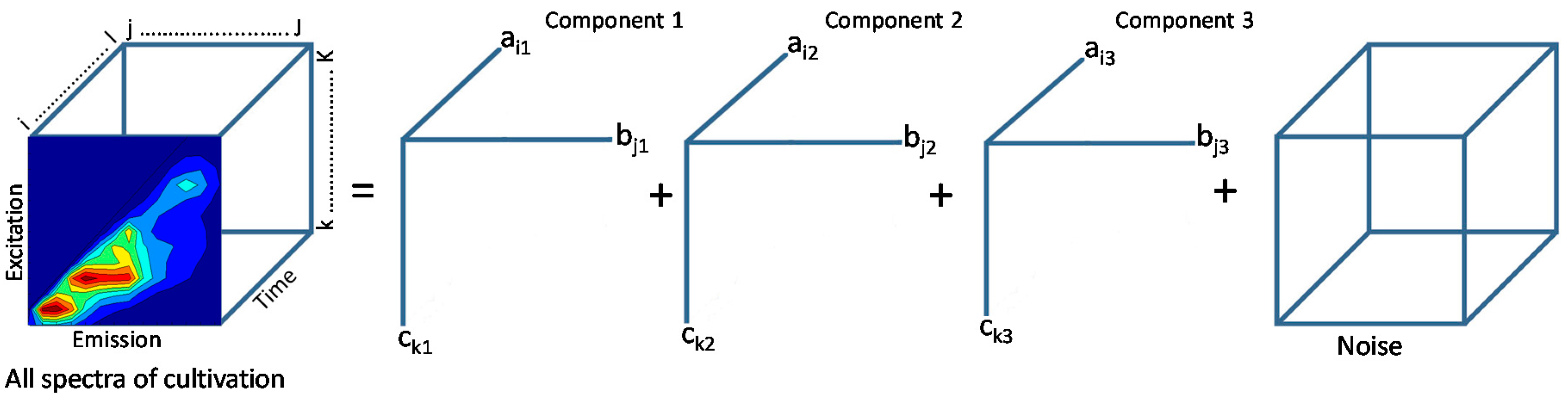
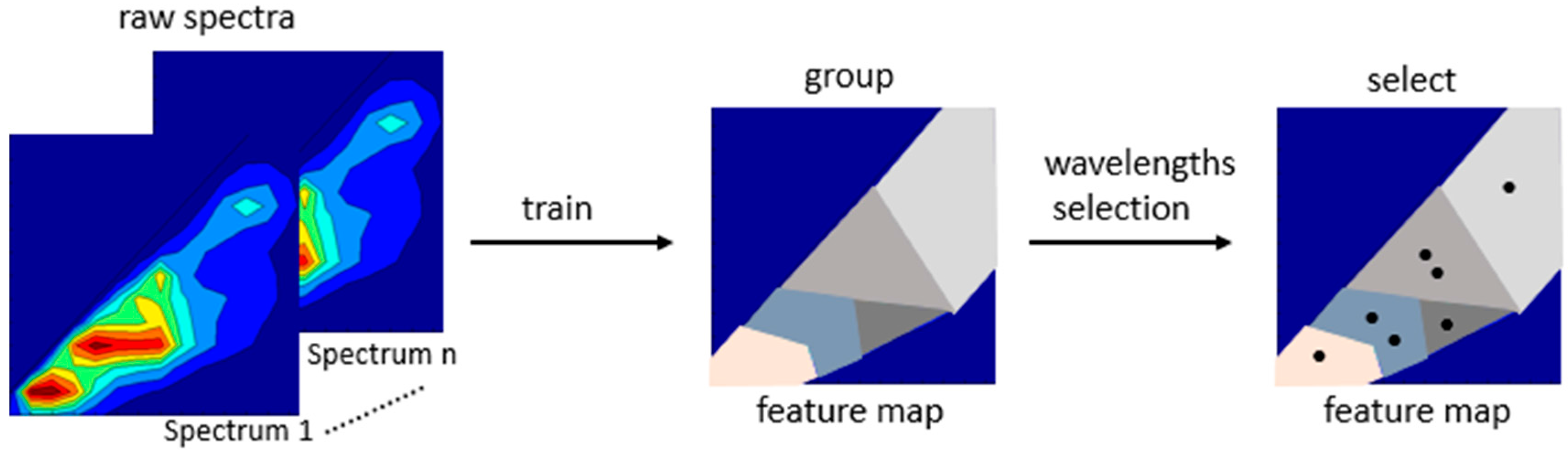
3.3. Modeling for Variable Prediction
4. Conclusions and Future Trends
| Method | Application | Evaluation | Software | Ref. |
|---|---|---|---|---|
| PLS | Glycoprotein yield prediction | Relative errors: 2.3%–4.6% | MATLAB® | [30] |
| Glycerol/methanol prediction | Mean prediction errors: 7%–10% | Unscrambler® | [51] | |
| Biomass/polymixin prediction | RMSECV: biomass 0.4 g/L, polymixin 35 mg/L | Unscrambler® | [42] | |
| Biomass, glucose, ethanol and product prediction | R2: biomass 0.53, glucose: 0.88 | MATLAB® | [55] | |
| OD, glycerol and 1,3-propanediol prediction | ethanol 0.01, product 0.73 | |||
| Biomass, glucose, CPR | RMSEP: OD 0.78 units, | MATLAB® | [43] | |
| glycerol 10 g/L, 1,3-PD 2.6 g/L | ||||
| Cell density and glycoprotein | RMSEP: biomass (three conditions) 3.9%–40.7%, glucose 6.8%, CPR 9.1% | Unscrambler® | [33] | |
| in 95% confidence interval, R2 = 0.91 cell density, 0.99 glycoprotein | ||||
| Biomass and glycerol | RMSEP: biomass 0.67/0.729 glycerol 1.52/0.911 | - | [56] | |
| Total amino acids, biomass | RMSECV: CDW 1.02 g/L , AA 1.06 g/L | |||
| Cell count (CC), OD, po2% | RMSECV: CC 1.029, OD 0.046, pO2% 5.358 R2: CC 0.936, OD 0.988, pO2% 0.977 | MATLAB® | [48] | |
| RMSEP: ALA 38.512 mg/L DO 5.1506% | MATLAB® | [28] | ||
| Extracellular 5-aminolevulinic acid (ALA), disolved oxygen (DO), CO2 | CO2 0.756% | MATLAB® | [54] | |
| Biomass, protein, alkaloid | RMSEP: biomass 7.26%, proteins 5.74%, | Unscrambler® | ||
| alkaloids 3.37% | MATLAB® | [36] | ||
| Glucose, lactate, glutamine | RMSEP: glucose 0.524 g/L, lactate 0.494 g/L | |||
| glutamate 0.0155 g/L R2: glucose 0.967, | Unscrambler® | [8] | ||
| lactate 0.972, glutamate 0.983 | ||||
| Cellmass, lipase activity | R2: cellmass 0.73–0.97, lipase activity 0.93 | Unscrambler® | [57] | |
| RMSECV cellmass 0.77–1.48 g/kg | ||||
| Biomass | RMSEP: 4.6 g/L | |||
| Biomass, ethanol, glucose | RMSEP: 4%, 2%–8%, 4% | MATLAB® | [44] | |
| Regulation of optimal feed | - | |||
| Biomass, glucose | - | MATLAB® | [29] | |
| Biomass | RMSEP: 0.19 g/L (PLS), | MATLAB® | [10] | |
| pH-value, acidity | RMSEP: 2.36%–4.84%, 6.04%–8.08% | Unscrambler® | [11] | |
| Enzyme activity | RMSEP: 0.08–0.12 | MATLAB® | [25] | |
| MATLAB® | [52] | |||
| MATLAB® | [69] | |||
| MATLAB® | [47] | |||
| PCA | Plasmid containing strain stability | - | SIMCA-P 8.0 | [32] |
| Medium wash steps, cell growth | - | Mathematica | [46] | |
| Cultivation description with scores | - | MATLAB® | [36,37] | |
| PCR | Extracellular 5-aminolevulinic acid (ALA), disolved oxygen (DO), CO2 | RMSEP: ALA 38.344 mg/L DO 5.296% | MATLAB® | [36] |
| CO2 1.225% | ||||
| pH-value, acidity | RMSEP: 3.60%–5.10%, 6.45%–9.97% | MATLAB® | [69] | |
| Linear regression Linear regression | Biomass prediction | R2 = 0.9869 | - | [41] |
| Biomass and PHB prediction | linear correlation to NADH signal | - | [20] | |
| Biomass | MARE = 0.12 | MATLAB® | [49] | |
| Biomass | R2 = 0.91 | - | [59] | |
| Total amino acids, biomass | RMSECV: CDW 1.18 g/L, AA 0.80 g/L | MATLAB® | [28] | |
| NPLS | Estimation of product yield | RMSEV: 0.13 g/L | MATLAB® | [58] |
| Enzyme activity | RMSEP: 0.08–0.12 | MATLAB® | [47] | |
| Total amino acids, biomass | RMSECV: CDW 1.39 g/L, AA 2.17 g/L | MATLAB® | [28] | |
| Biomass | RMSEP: 5%–7% | MATLAB® | [10] | |
| PARAFAC | Cultivation description | - | MATLAB® | [44] |
| Biomass | RMSEP: 0.20 g/L | MATLAB® | [52] | |
| Luedeking-Piret-based equation | Biomass | MARE = 0.06 | MATLAB® | [49] |
| ANN | 3-Chloro-4-methylaniline | R2 > 0.7 | microCortex | [26] |
| pH value, acidity | RMSEP: 2.44%–3.42% , 6.89–12.11 | MATLAB® | [69] | |
| FFNN | Biomass, glucose | R2: glucose 0.88, biomass 0.93 Largest observed error: biomass 1 g/L, glucose 8 g/L | MATLAB® | [25] |
| BPNN | Biomass, glucose, CO2, DO, O2, | evaluation of BPNN topology all Rxy > 0.97 | MATLAB® | [35] |
| Total amino acids | RMSEP 0.112–0.165 g/L | |||
| RBF | Biomass (BDM), total cell number (TCN), dead cells (DC), product, plasmid copy number (PCN) | BDM 0.5 g/L, TCN 17 1/mL, DC 1% | MATLAB® | [9] |
| Product 7 mg/g BDM, PCN 8 units |
Conflicts of Interest
References
- Warth, B.; Rajkai, G.; Mandenius, C.F. Evaluation of software sensors for on-line estimation of culture conditions in an Escherichia coli cultivation expressing a recombinant protein. J. Biotechnol. 2010, 147, 37–45. [Google Scholar] [CrossRef]
- Gnoth, S.; Jenzsch, M.; Simutis, R.; Lübbert, A. Process Analytical Technology (PAT): Batch-to-batch reproducibility of fermentation processes by robust process operational design and control. J. Biotechnol. 2007, 132, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Costa, P.F.; Alves, T.P.; Menezes, J.C. Chemometrics in bioprocess engineering: process analytical technology (PAT) applications. Chemom. Intell. Lab. Syst. 2004, 74, 269–275. [Google Scholar] [CrossRef]
- Rathore, A.S.; Winkle, H. Quality by design for biopharmaceuticals. Nat. Biotechnol. 2009, 27, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Tohmola, N.; Ahtinen, J.; Pitkänen, J.P.; Parviainen, V.; Joenväärä, S.; Hautamäki, M.; Lindroos, P.; Mäkinen, J.; Renkonen, R. On-line high performance liquid chromatography measurements of extracellular metabolites in an aerobic batch yeast (Saccharomyces cerevisiae) culture. Biotechnol. Bioprocess Eng. 2011, 16, 264–272. [Google Scholar] [CrossRef]
- Klockow, C.; Hüll, D.; Hitzmann, B. Model based substrate set point control of yeast cultivation processes based on FIA measurements. Anal. Chim. Acta 2008, 623, 30–37. [Google Scholar] [CrossRef]
- Weigel, B.; Hitzmann, B.; Kretzmer, G.; Schügerl, K.; Huwig, A.; Giffhorn, F. Analysis of various sugars by means of immobilized enzyme coupled flow injection analysis. J. Biotechnol. 1996, 50, 93–106. [Google Scholar] [CrossRef]
- Boehl, D.; Solle, D.; Hitzmann, B.; Scheper, T. Chemometric modelling with two-dimensional fluorescence data for Claviceps purpurea bioprocess characterization. J. Biotechnol. 2003, 105, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Clementschitsch, F.; Jürgen, K.; Florentina, P.; Karl, B. Sensor combination and chemometric modelling for improved process monitoring in recombinant E. coli fed-batch cultivations. J. Biotechnol. 2005, 120, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Ödman, P.; Johansen, C.L.; Olsson, L.; Gernaey, K.V.; Lantz, A.E. On-line estimation of biomass, glucose and ethanol in Saccharomyces cerevisiae cultivations using in-situ multi-wavelength fluorescence and software sensors. J. Biotechnol. 2009, 144, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Hantelmann, K.; Kollecker, M.; Hüll, D.; Hitzmann, B.; Scheper, T. Two-dimensional fluorescence spectroscopy: A novel approach for controlling fed-batch cultivations. J. Biotechnol. 2006, 121, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Sandor, M.; Rudinger, F.; Solle, D.; Bienert, R.; Grimm, C.; Grosz, S.; Scheper, T. NIR-spectroscopy for bioprocess monitoring & control. BMC Proc. 2013, 7. [Google Scholar] [CrossRef]
- Scarff, M.; Arnold, S.A.; Harvey, L.M.; McNeil, B. Near Infrared Spectroscopy for Bioprocess Monitoring and Control: Current Status and Future Trends. Crit. Rev. Biotechnol. 2006, 26, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Cervera, A.E.; Petersen, N.; Lantz, A.E.; Larsen, A.; Gernaey, K.V. Application of near-infrared spectroscopy for monitoring and control of cell culture and fermentation. Biotechnol. Prog. 2009, 25, 1561–1581. [Google Scholar] [PubMed]
- Moretto, J.; Smelko, J.P.; Cuellar, M.; Berry, B.; Doane, A.; Ryll, T.; Wiltberger, K. Process Raman Spectroscopy for In-Line CHO Cell Culture Monitoring. Am. Pharm. Rev. 2011, 14, 18–25. [Google Scholar]
- Oh, S.K.; Yoo, S.J.; Jeong, D.H.; Lee, J.M. Real-time estimation of glucose concentration in algae cultivation system using Raman spectroscopy. Bioresour. Technol. 2013, 142, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mercier, S.M.; Diepenbroek, B.; Wijffels, R.H.; Streefland, M. Multivariate PAT solutions for biopharmaceutical cultivation: Current progress and limitations. Trends Biotechnol. 2014, 32, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.F.; Chance, B. Fluorimetric Technique for Monitoring Changes in the Level of Reduced Nicotinamide Nucleotides in Continuous Cultures of Microorganisms. Appl. Microbiol. 1970, 19, 446–450. [Google Scholar] [PubMed]
- Zabriskie, D.W.; Humphrey, A.E. Estimation of Fermentation Biomass Concentration by Measuring Culture Fluorescence. Appl. Environ. Microbiol. 1978, 35, 337–343. [Google Scholar] [PubMed]
- Gahlawat, G.; Srivastava, A. Use of NAD(P)H Fluorescence Measurement for On-Line Monitoring of Metabolic State of Azohydromonas australica in Poly(3-hydroxybutyrate) Production. Appl. Biochem. Biotechnol. 2013, 169, 821–831. [Google Scholar] [CrossRef]
- Beutel, S.; Henkel, S. In situ sensor techniques in modern bioprocess monitoring. Appl. Microbiol. Biotechnol. 2011, 91, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Humphrey, A.E. Use of fluorometry for monitoring and control of a bioreactor. Biotechnol. Bioeng. 1991, 37, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Hagedorn, A.; Legge, R.L.; Budman, H. Evaluation of spectrofluorometry as a tool for estimation in fed-batch fermentations. Biotechnol. Bioeng. 2003, 83, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Almeida, J.S.; Crespo, J.G.; Reis, M.A. An improved method for two-dimensional fluorescence monitoring of complex bioreactors. J. Biotechnol. 2007, 128, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.I.; Lee, K.I.; Kim, C.K.; Yim, Y.S.; Chung, S.W.; Wei, J.; Bellgardt, K.H. Classification of two-dimensional fluorescence spectra using self-organizing maps. Biochem. Eng. J. 2005, 22, 135–144. [Google Scholar] [CrossRef]
- Ödman, P.; Johansen, C.; Olsson, L.; Gernaey, K.; Lantz, A. Sensor combination and chemometric variable selection for online monitoring of Streptomyces coelicolor fed-batch cultivations. Appl. Microbiol. Biotechnol. 2010, 86, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Rønnest, N.; Stocks, S.; Eliasson Lantz, A.; Gernaey, K. Introducing process analytical technology (PAT) in filamentous cultivation process development: Comparison of advanced online sensors for biomass measurement. J. Ind. Microbiol. Biotechnol. 2011, 38, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shanahan, M.; Calvet, A.; Leister, K.J.; Ryder, A.G. Comprehensive, quantitative bioprocess productivity monitoring using fluorescence EEM spectroscopy and chemometrics. Analyst 2014, 139, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Ranzan, C.; Strohm, A.; Ranzan, L.; Trierweiler, L.F.; Hitzmann, B.; Trierweiler, J.O. Wheat flour characterization using NIR and spectral filter based on Ant Colony Optimization. Chemom. Intell. Lab. Syst. 2014, 132, 133–140. [Google Scholar] [CrossRef]
- Johansson, L.; Lidén, G. A Study of Long-Term Effects on Plasmid-Containing Escherichia coli in Carbon-Limited Chemostat Using 2D-Fluorescence Spectrofluorimetry. Biotechnol. Prog. 2006, 22, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Jayaraman, G.; Kökpinar, Ö.; Rinas, U.; Hitzmann, B. On-line monitoring of recombinant bacterial cultures using multi-wavelength fluorescence spectroscopy. Biochem. Eng. J. 2011, 58, 133–139. [Google Scholar] [CrossRef]
- Samorski, M.; Müller-Newen, G.; Büchs, J. Quasi-continuous combined scattered light and fluorescence measurements: A novel measurement technique for shaken microtiter plates. Biotechnol. Bioeng. 2005, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Yim, Y.S.; Chung, S.W.; Wei, J.; Rhee, J.I. Application of artificial neural networks to the analysis of two-dimensional fluorescence spectra in recombinant E coli fermentation processes. J. Chem. Technol. Biotechnol. 2005, 80, 1036–1045. [Google Scholar] [CrossRef]
- Rhee, J.I.; Kang, T.H. On-line process monitoring and chemometric modeling with 2D fluorescence spectra obtained in recombinant E. coli fermentations. Process Biochem. 2007, 42, 1124–1134. [Google Scholar] [CrossRef]
- Rhee, J.; Kang, T.H.; Lee, K.I.; Sohn, O.J.; Kim, S.Y.; Chung, S.W. Application of principal component analysis and self-organizing map to the analysis of 2D fluorescence spectra and the monitoring of fermentation processes. Biotechnol. Bioprocess Eng. 2006, 11, 432–441. [Google Scholar] [CrossRef]
- Ju, L.K.; Chen, F.; Xia, Q. Monitoring microaerobic denitrification of Pseudomonas aeruginosa by online NAD(P)H fluorescence. J. Ind. Microbiol. Biotechnol. 2005, 32, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.F.; Trauthwein, H.; Dingerdissen, U.; Rieping, M.; Huthmacher, K. Monitoring aerobic Escherichia coli growth in shaken microplates by measurement of culture fluorescence. Biotechniques 2004, 36, 580–582. [Google Scholar] [PubMed]
- Kensy, F.; Zang, E.; Faulhammer, C.; Tan, R.K.; Buchs, J. Validation of a high-throughput fermentation system based on online monitoring of biomass and fluorescence in continuously shaken microtiter plates. Microb. Cell Fact. 2009, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, S.; Srivastava, A. On-line Characterization of Physiological State in Poly(β-Hydroxybutyrate) Production by Wautersia eutropha. Appl. Biochem. Biotechnol. 2009, 157, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Eliasson Lantz, A.; Jørgensen, P.; Poulsen, E.; Lindemann, C.; Olsson, L. Determination of cell mass and polymyxin using multi-wavelength fluorescence. J. Biotechnol. 2006, 121, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Solle, D.; Hitzmann, B.; Ayub, M. Chemometric modeling and two-dimensional fluorescence analysis of bioprocess with a new strain of Klebsiella pneumoniae to convert residual glycerol into 1,3-propanediol. J. Ind. Microbiol. Biotechnol. 2012, 39, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.B.; Lantz, A.E.; Mortensen, P.P.; Olsson, L. Chemometric analysis of in-line multi-wavelength fluorescence measurements obtained during cultivations with a lipase producing Aspergillus oryzae strain. Biotechnol. Bioeng. 2007, 96, 904–913. [Google Scholar] [CrossRef]
- Ganzlin, M.; Marose, S.; Lu, X.; Hitzmann, B.; Scheper, T.; Rinas, U. In situ multi-wavelength fluorescence spectroscopy as effective tool to simultaneously monitor spore germination, metabolic activity and quantitative protein production in recombinant Aspergillus niger fed-batch cultures. J. Biotechnol. 2007, 132, 461–468. [Google Scholar] [CrossRef]
- Jhala, E.; Galilee, C.; Reinisch, L. Principal component analysis of fluorescence changes upon growth conditions and washing of Pseudomonas aeruginosa. Appl. Opt. 2007, 46, 5522–5528. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.P.; Bro, R. Real-time monitoring and chemical profiling of a cultivation process. Chemom. Intell. Lab. Syst. 2006, 84, 106–113. [Google Scholar] [CrossRef]
- Surribas, A.; Amigo, J.; Coello, J.; Montesinos, J.; Valero, F.; Maspoch, S. Parallel factor analysis combined with PLS regression applied to the on-line monitoring of Pichia pastoris cultures. Anal. Bioanal. Chem. 2006, 385, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Surribas, A.; Montesinos, J.L.; Valero, F.F. Biomass estimation using fluorescence measurements in Pichia pastoris bioprocess. J. Chem. Technol. Biotechnol. 2006, 81, 23–28. [Google Scholar]
- Hisiger, S.; Jolicoeur, M. A multiwavelength fluorescence probe: Is one probe capable for on-line monitoring of recombinant protein production and biomass activity? J. Biotechnol. 2005, 117, 325–336. [Google Scholar] [CrossRef]
- Surribas, A.; Geissler, D.; Gierse, A.; Scheper, T.; Hitzmann, B.; Montesinos, J.L.; Valero, F. State variables monitoring by in situ multi-wavelength fluorescence spectroscopy in heterologous protein production by Pichia pastoris. J. Biotechnol. 2006, 124, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.B.; Eliasson, A.; Olsson, L. On-line cell mass monitoring of Saccharomyces cerevisiae cultivations by multi-wavelength fluorescence. J. Biotechnol. 2004, 114, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Masiero, S.S.; Trierweiler, J.O.; Farenzena, M.; Escobar, M.; Trierweiler, L.F.; Ranzan, C. Evaluation of wavelength selection methods for 2D fluorescence spectra applied to bioprocesses characterization. Braz. J. Chem. Eng. 2013, 30, 289–298. [Google Scholar] [CrossRef]
- Bogomolov, A.; Grasser, T.; Hessling, M. In-line monitoring of Saccharomyces cerevisiae fermentation with a fluorescence probe: New approaches to data collection and analysis. J. Chemom. 2011, 25, 389–399. [Google Scholar] [CrossRef]
- Hagedorn, A.; Levadoux, W.; Groleau, D.; Tartakovsky, B. Evaluation of Multiwavelength Culture Fluorescence for Monitoring the Aroma Compound 4-Hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF) Production. Biotechnol. Prog. 2004, 20, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.P.; Portugal, C.A.; Carinhas, N.; Dias, J.M.L.; Crespo, J.P.; Alves, P.M.; Carrondo, M.J.T.; Oliveira, R. In situ 2D fluorometry and chemometric monitoring of mammalian cell cultures. Biotechnol. Bioeng. 2009, 102, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Bonk, S.; Sandor, M.; Rudinger, F.; Tscheschke, B.; Prediger, A.; Babitzky, A.; Solle, D.; Beutel, S.; Scheper, T. In-situ microscopy and 2D fluorescence spectroscopy as online methods for monitoring CHO cells during cultivation. BMC Proc. 2011, 5 (Suppl 8), 76. [Google Scholar] [CrossRef]
- Ryan, P.W.; Li, B.; Shanahan, M.; Leister, K.J.; Ryder, A.G. Prediction of cell culture media performance using fluorescence spectroscopy. Anal. Chem. 2010, 82, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Harsh, S.; Srivastava, A.K. Use of NADH fluorescence measurement for on-line biomass estimation and characterization of metabolic status in bioreactor cultivation of plant cells for azadirachtin (a biopesticide) production. Process Biochem. 2008, 43, 1121–1123. [Google Scholar] [CrossRef]
- Hisiger, S.; Jolicoeur, M. Plant Cell Culture Monitoring Using an in situ Multiwavelength Fluorescence Probe. Biotechnol. Prog. 2005, 21, 580–589. [Google Scholar] [CrossRef]
- Ulber, R.; Protsch, C.; Solle, D.; Hitzmann, B.; Willke, B.; Faurie, R.; Scheper, T. Use of Bioanalytical Systems for the Improvement of Industrial Tryptophan Production. Eng. Life Sci. 2001, 1, 15–17. [Google Scholar] [CrossRef]
- Tsien, R.Y. The green fluorescent protein. Ann. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia 2000, 2, 89–117. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.P.; Mutharasan, R. Inner filter effects and their interferences in the interpretation of culture fluorescence. Biotechnol. Bioeng. 1987, 30, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2005, 2, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Schulmann, S.G. Molecular Luminescence Spectroscopy Methods and Applications—Part 1; John Wiley and Sons: New York, NY, USA, 1985. [Google Scholar]
- Pundak, S.; Roche, R.S. Tyrosine and tyrosinate fluorescence of bovine testes calmodulin: Calcium and pH dependence. Biochemistry 1984, 23, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Fluorophores. Principles of Fluorescence Spectroscopy; Lakowicz, J., Ed.; Springer US: New York, NY, USA, 2006; pp. 63–95. [Google Scholar]
- Grote, B.; Zense, T.; Hitzmann, B. 2D-fluorescence and multivariate data analysis for monitoring of sourdough fermentation process. Food Control 2014, 38, 8–18. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Bro, R. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Kang, C.; Wu, H.L.; Xiang, S.X.; Xie, L.X.; Liu, Y.J.; Yu, Y.J.; Sun, J.J.; Yu, R.Q. Simultaneous determination of aromatic amino acids in different systems using three-way calibration based on the PARAFAC-ALS algorithm coupled with EEM fluorescence: Exploration of second-order advantages. Anal. Methods 2014, 6, 6358–6368. [Google Scholar] [CrossRef]
- Andersen, C.M.; Bro, R. Practical aspects of PARAFAC modeling of fluorescence excitation-emission data. J. Chemom. 2003, 17, 200–215. [Google Scholar] [CrossRef]
- Kohonen, T. The self-organizing map. IEEE Proc. 1990, 78, 1464–1480. [Google Scholar] [CrossRef]
- Leardi, R. Genetic algorithms in chemometrics and chemistry: A review. J. Chemom. 2001, 15, 559–569. [Google Scholar] [CrossRef]
- Jouan-Rimbaud, D.; Massart, D.L.; Leardi, R.; De Noord, O.E. Genetic Algorithms as a Tool for Wavelength Selection in Multivariate Calibration. Anal. Chem. 1995, 67, 4295–4301. [Google Scholar] [CrossRef]
- Allegrini, F.; Olivieri, A.C. A new and efficient variable selection algorithm based on ant colony optimization. Applications to near infrared spectroscopy/partial least-squares analysis. Anal. Chim. Acta 2011, 699, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bro, R. Multiway calibration. Multilinear PLS. J. Chemom. 1996, 10, 47–61. [Google Scholar] [CrossRef]
- Liu, R.X.; Kuang, J.; Gong, Q.; Hou, X.L. Principal component regression analysis with spss. Comput. Methods Programs Biomed. 2003, 71, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.A.; Bro, R. The N-way Toolbox for MATLAB. Chemom. Intell. Lab. Syst. 2000, 52, 1–4. [Google Scholar] [CrossRef]
- Wolf, G.; Almeida, J.S.; Pinheiro, C.; Correia, V.; Rodrigues, C.; Reis, M.A.; Crespo, J.G. Two-dimensional fluorometry coupled with artificial neural networks: A novel method for on-line monitoring of complex biological processes. Biotechnol. Bioeng. 2001, 72, 297–306. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Legge, R.; Budman, H. Comparative study of black-box and hybrid estimation methods in fed-batch fermentation. J. Process Control 2002, 12, 113–121. [Google Scholar] [CrossRef]
- Hornik, K.; Stinchcombe, M.; White, H. Multilayer feedforward networks are universal approximators. Neural Netw. 1989, 2, 359–366. [Google Scholar] [CrossRef]
- Planck, M. Wissenschaftliche Selbstbiographie. Mit einem Bildnis und der von Max von Laue gehaltenen Traueransprache; Aufl.; Barth: Leipzig, Germany, 1948. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faassen, S.M.; Hitzmann, B. Fluorescence Spectroscopy and Chemometric Modeling for Bioprocess Monitoring. Sensors 2015, 15, 10271-10291. https://doi.org/10.3390/s150510271
Faassen SM, Hitzmann B. Fluorescence Spectroscopy and Chemometric Modeling for Bioprocess Monitoring. Sensors. 2015; 15(5):10271-10291. https://doi.org/10.3390/s150510271
Chicago/Turabian StyleFaassen, Saskia M., and Bernd Hitzmann. 2015. "Fluorescence Spectroscopy and Chemometric Modeling for Bioprocess Monitoring" Sensors 15, no. 5: 10271-10291. https://doi.org/10.3390/s150510271





