Use of a Modified Vector Model for Odor Intensity Prediction of Odorant Mixtures
Abstract
:1. Introduction
2. Experimental Section
2.1. Odor Sample and Assessors
| Order | Odorant | Abbreviation | CAS# | ChemicalStructure | Odor Threshold/(mg/m3) |
|---|---|---|---|---|---|
| 1 | Benzene | B | 71-43-2 |  | 2.53 |
| 2 | Toluene | T | 108-88-3 |  | 1.43 |
| 3 | Ethylbenzene | E | 100-41-4 |  | 0.45 |
| 4 | n-Propylbenzene | P | 103-65-7 |  | 0.57 |
| 5 | o-Xylene | O | 95-47-6 |  | 1.37 |
| 6 | m-Xylene | M | 108-38-3 |  | 1.55 |
| 7 | Styrene | S | 100-42-5 |  | 0.19 |
2.2. The Vector Model Methodology
2.3. Experimental Procedure
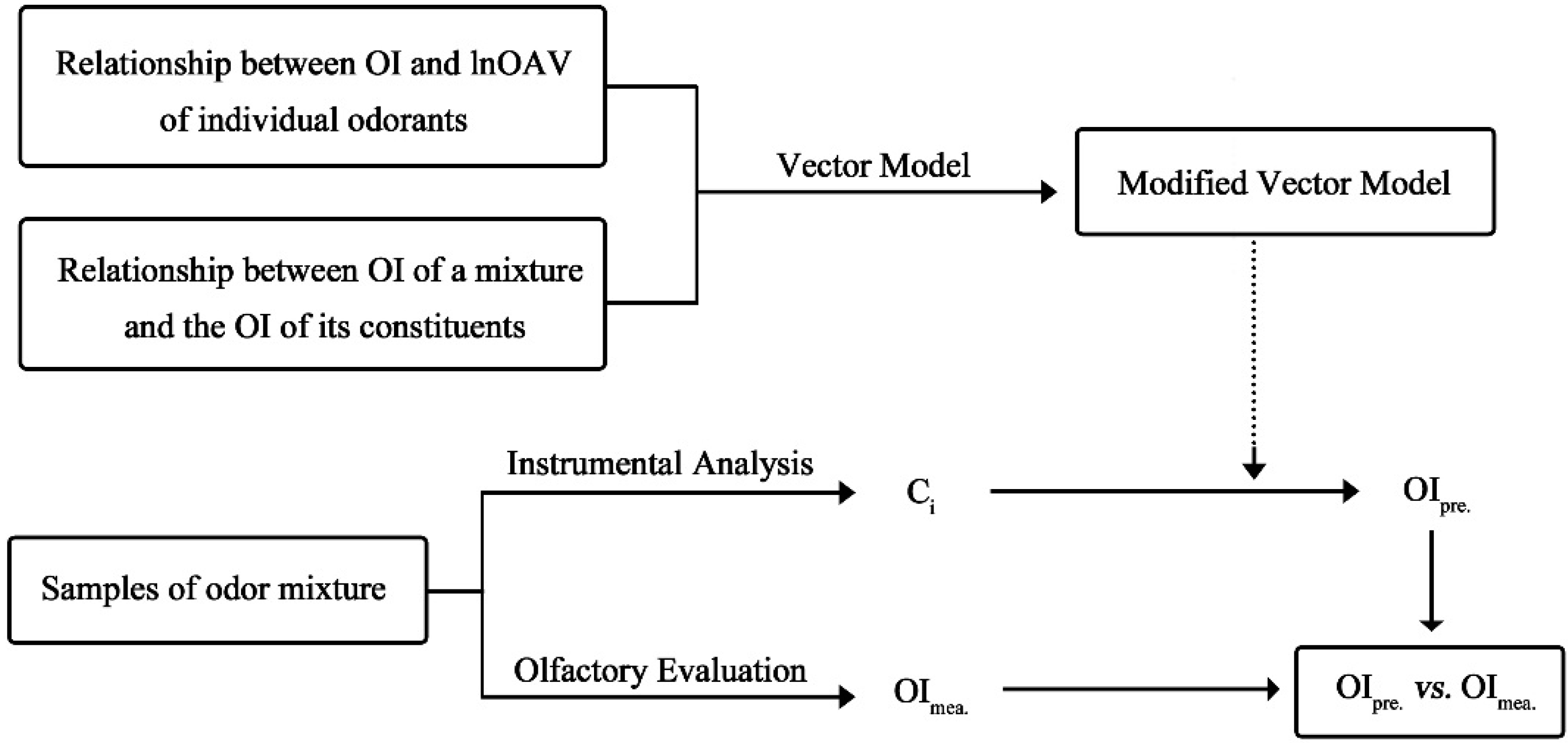
2.4. Olfactory and Chemical Analysis
3. Results and Discussion
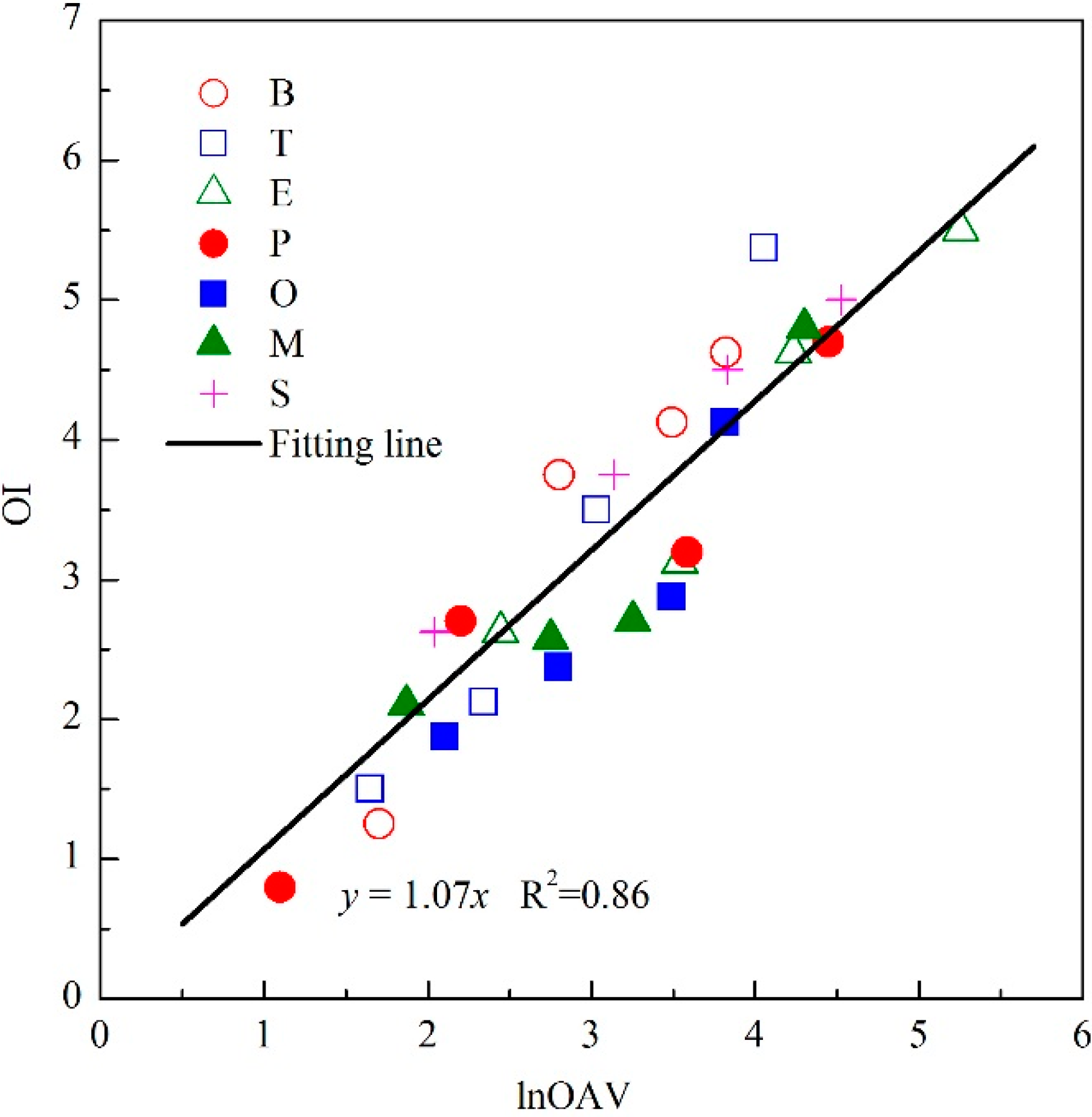
3.1. The Linear OI-lnOAV Relation of Individual Odorants
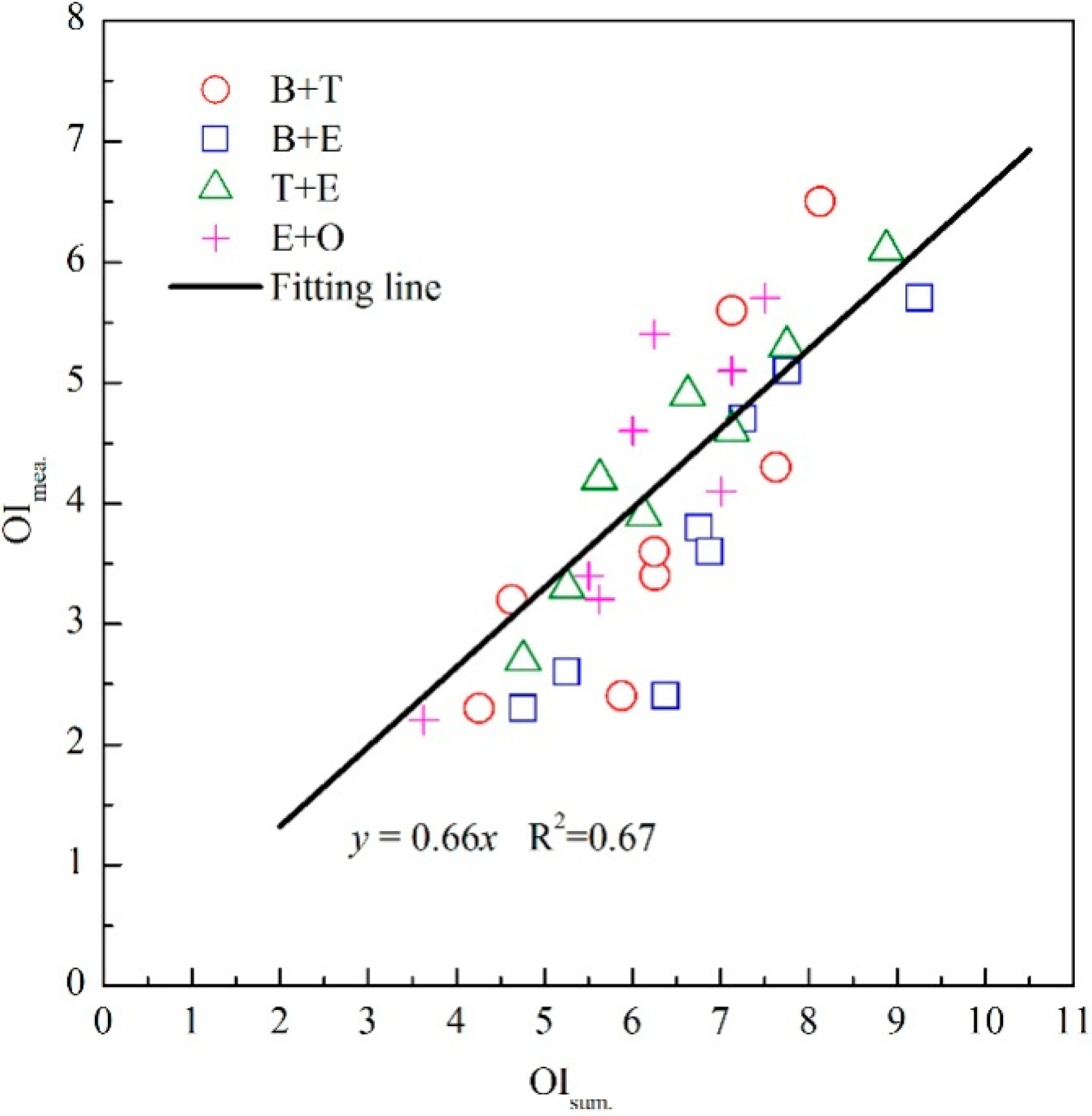
3.2. The Modification of Vector Model for Binary Odor Mixture
3.3. The Identification of Modified Vector Model in OI Prediction

| Odor Mixture | Concentration of Each Component | OImea. | OIpre. | ||||
|---|---|---|---|---|---|---|---|
| lnOAVa | lnOAVb | lnOAVc | lnOAVd | MVMI | SCMII | ||
| T (a) + E (b) | 3.36 | 3.54 | - | - | 5.4 | 4.9 | 3.8 |
| 3.03 | 2.44 | - | - | 4.0 | 3.9 | 3.2 | |
| 2.67 | 2.44 | - | - | 3.3 | 3.6 | 2.9 | |
| T (a) + S (b) | 1.65 | 2.04 | - | - | 2.7 | 2.6 | 2.2 |
| 3.36 | 2.04 | - | - | 4.5 | 4.0 | 3.6 | |
| 2.34 | 4.53 | - | - | 6.2 | 5.2 | 4.8 | |
| T (a) + E (b) + M (c) | 2.34 | 3.54 | 2.80 | - | 3.8 | 4.7 | 3.8 |
| 2.34 | 2.44 | 3.49 | - | 5.0 | 4.5 | 3.7 | |
| 1.65 | 1.78 | 2.15 | - | 3.5 | 3.0 | 2.3 | |
| E (a) + P (b) + S (c) | 3.54 | 2.20 | 3.83 | - | 5.4 | 5.3 | 4.1 |
| 4.24 | 2.20 | 4.53 | - | 6.8 | 6.2 | 4.8 | |
| 2.82 | 2.20 | 2.65 | - | 4.5 | 4.1 | 3.0 | |
| T (a) + E (b) + M (c) + S (d) | 4.24 | 2.20 | 3.83 | 2.15 | 4.8 | 5.5 | 4.5 |
| 2.20 | 3.15 | 2.20 | 2.15 | 4.5 | 4.1 | 3.4 | |
| 1.85 | 1.77 | 2.20 | 2.15 | 3.5 | 3.3 | 2.4 | |
| Average of OIpre./OImea. | 0.96 | 0.78 | |||||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, A.; Singh, B.P.; Punia, M.; Singh, D.; Kumar, K.; Jain, V.K. Assessment of indoor air concentrations of VOCs and their associated health risks in the library of Jawaharlal Nehru University, New Delhi. Environ. Sci. Pollut. Res. Int. 2014, 21, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Brodzik, K.; Faber, J.; Lomankiewicz, D.; Golda-Kopek, A. In-vehicle VOCs composition of unconditioned, newly produced cars. J. Environ. Sci. 2014, 26, 1052–1061. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Yan, L.; Chen, H.; Shao, H.; Meng, T. Assessment of odor activity value coefficient and odor contribution based on binary interaction effects in waste disposal plant. Atmos. Environ. 2015, 103, 231–237. [Google Scholar] [CrossRef]
- Abraham, M.H.; Sanchez-Moreno, R.; Cometto-Muniz, J.E.; Cain, W.S. An algorithm for 353 odor detection thresholds in humans. Chem. Senses 2012, 37, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, E.; Beno, N.; Ishii, A.; Chabanet, C.; Etievant, P.; Thomas-Danguin, T. Just noticeable differences in component concentrations modify the odor quality of a blending mixture. Chem. Senses 2008, 33, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Raeppel, C.; Appenzeller, B.M.; Millet, M. Determination of seven pyrethroid biocides and their synergy in indoor air by thermal-desorption gas chromatography/mass spectrometry after sampling on Tenax TA (R) passive tubes. Talanta 2015, 131, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Omur-Ozbek, P.; Gallagher, D.L.; Dietrich, A.M. Determining human exposure and sensory detection of odorous compounds released during showering. Environ. Sci. Technol. 2011, 45, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Kim, K.H.; Ahn, J.W.; Hong, O.F.; Chang, Y.S. Offensive odorants released from stormwater catch basins (SCB) in an urban area. Chemosphere 2010, 81, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, Y.H. Composition of key offensive odorants released from fresh food materials. Atmos. Environ. 2014, 89, 443–452. [Google Scholar] [CrossRef]
- Gebicki, J.; Dymerski, T.; Rutkowski, S. Identification of odor of volatile organic compounds using classical sensory analysis and electronic nose techniques. Environ. Prot. Eng. 2014, 40, 103–116. [Google Scholar]
- Curren, J.; Snyder, C.L.; Abraham, S.; Suffet, I.H. Comparison of two standard odor intensity evaluation methods for odor problems in air or water. Water Sci. Technol. 2014, 69, 142–146. [Google Scholar] [CrossRef] [PubMed]
- ASTM. E544-10 Standard Practices for Referencing Suprathreshold Odor Intensity; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Rodrigues, A.E.; Teixeira, M.A.; Rodriguez, O. The perception of fragrance mixtures: A comparison of odor intensity models. AICHE J. 2010, 56, 1090–1106. [Google Scholar]
- Cain, W.S.; Schiet, F.T.; Olsson, M.J.; de Wijk, R.A. Comparison of models of odor interaction. Chem. Senses 1995, 20, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Rodriguez, O.; Gomes, P.; Mata, V.; Rodrigues, A.E. Perfume Engineering: Design, Performance and Classification, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 96–108. [Google Scholar]
- Laffort, P. Several models of suprathreshold quantitative olfactory interactionin humans applied to binary, ternary and quaternary mixtures. Chem. Senses 1982, 7, 153–174. [Google Scholar] [CrossRef]
- Berglund, B.; Berglund, U.; Lindvall, T.; Svensson, L.T. A quantitative principle of perceived intensity summation in odor mixtures. J. Exp. Psychol. 1973, 100, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Whelton, A.J.; Dietrich, A.M. Relationship between intensity, concentration, and temperature for drinking water odorants. Water Res. 2004, 38, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Liden, E.; Nordin, S.; Hogman, L.; Ulander, A.; Deniz, F.; Gunnarsson, A.G. Assessment of odor annoyance and its relationship to stimulus concentration and odor intensity. Chem. Senses 1998, 23, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, J.; Wang, G.; Wu, C. An odor interaction model of binary odorant mixtures by a partial differential equation method. Sensors 2014, 14, 12256–12270. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H. Experimental demonstration of masking phenomena between competing odorants via an air dilution sensory test. Sensors 2010, 10, 7287–7302. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H. The averaging effect of odorant mixing as determined by air dilution sensory tests: A case study on reduced sulfur compounds. Sensors 2011, 11, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Snitz, K.; Yablonka, A.; Weiss, T.; Frumin, I.; Khan, R.M.; Sobel, N. Predicting odor perceptual similarity from odor structure. PLoS Comput. Biol. 2013, 9, e1003184. [Google Scholar] [CrossRef] [PubMed]
- Cain, W.S.; Drexler, M. Scope and evaluation of odor counteraction and masking. Ann. N. Y. Acad. Sci. 1974, 237, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, J.; Qu, C.; Gu, X.; Zhao, X. Research on odor interaction between aldehyde compounds via a partial differential equation (PDE) model. Sensors 2015, 15, 2888–2901. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Liu, J.; Fang, D. Use of a Modified Vector Model for Odor Intensity Prediction of Odorant Mixtures. Sensors 2015, 15, 5697-5709. https://doi.org/10.3390/s150305697
Yan L, Liu J, Fang D. Use of a Modified Vector Model for Odor Intensity Prediction of Odorant Mixtures. Sensors. 2015; 15(3):5697-5709. https://doi.org/10.3390/s150305697
Chicago/Turabian StyleYan, Luchun, Jiemin Liu, and Di Fang. 2015. "Use of a Modified Vector Model for Odor Intensity Prediction of Odorant Mixtures" Sensors 15, no. 3: 5697-5709. https://doi.org/10.3390/s150305697





