A Bio-Inspired Two-Layer Sensing Structure of Polypeptide and Multiple-Walled Carbon Nanotube to Sense Small Molecular Gases
Abstract
:1. Introduction
2. Experiment
2.1. MWCNT-Polypeptide Gas Sensor
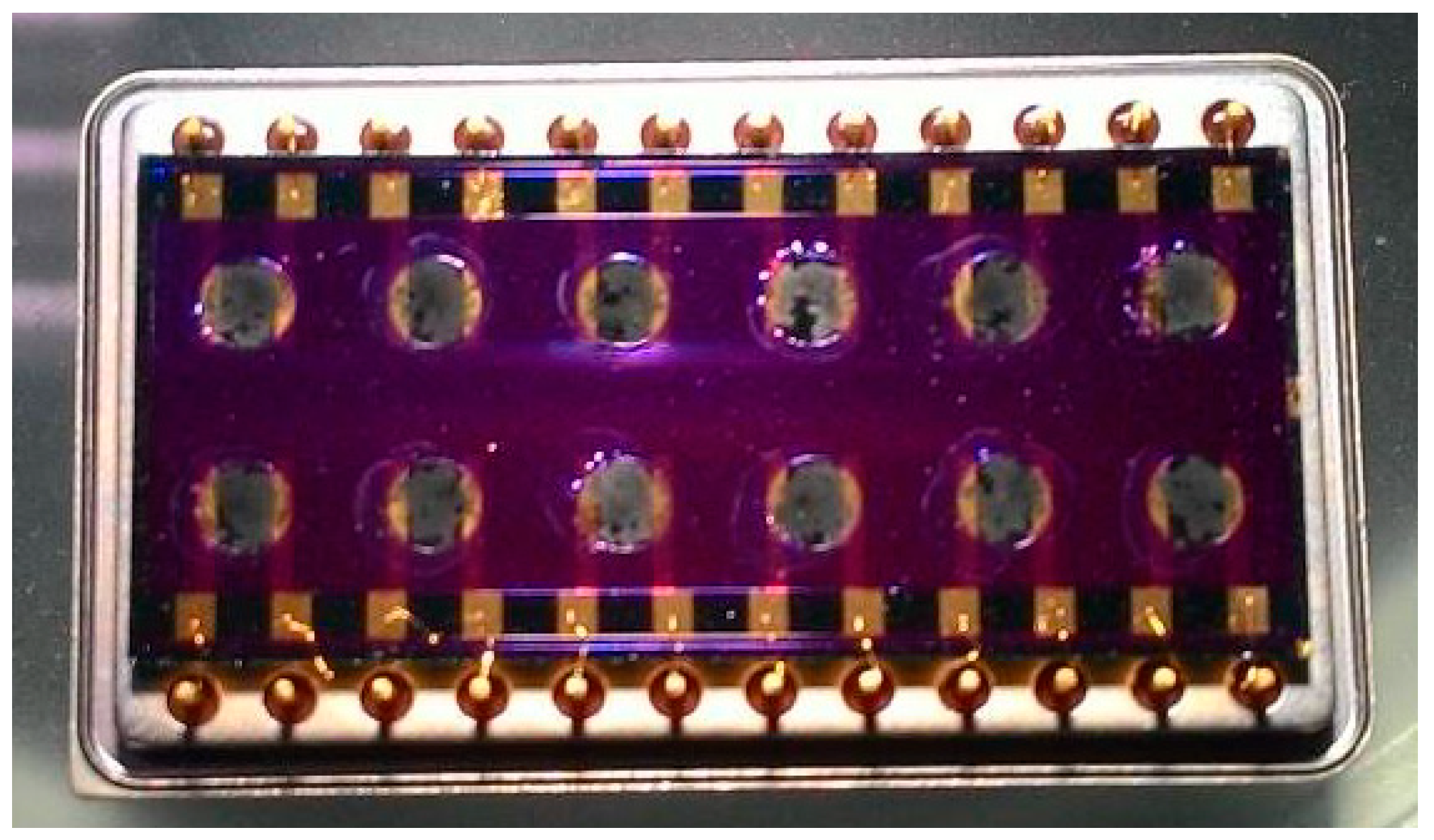
2.2. Experimental Setup
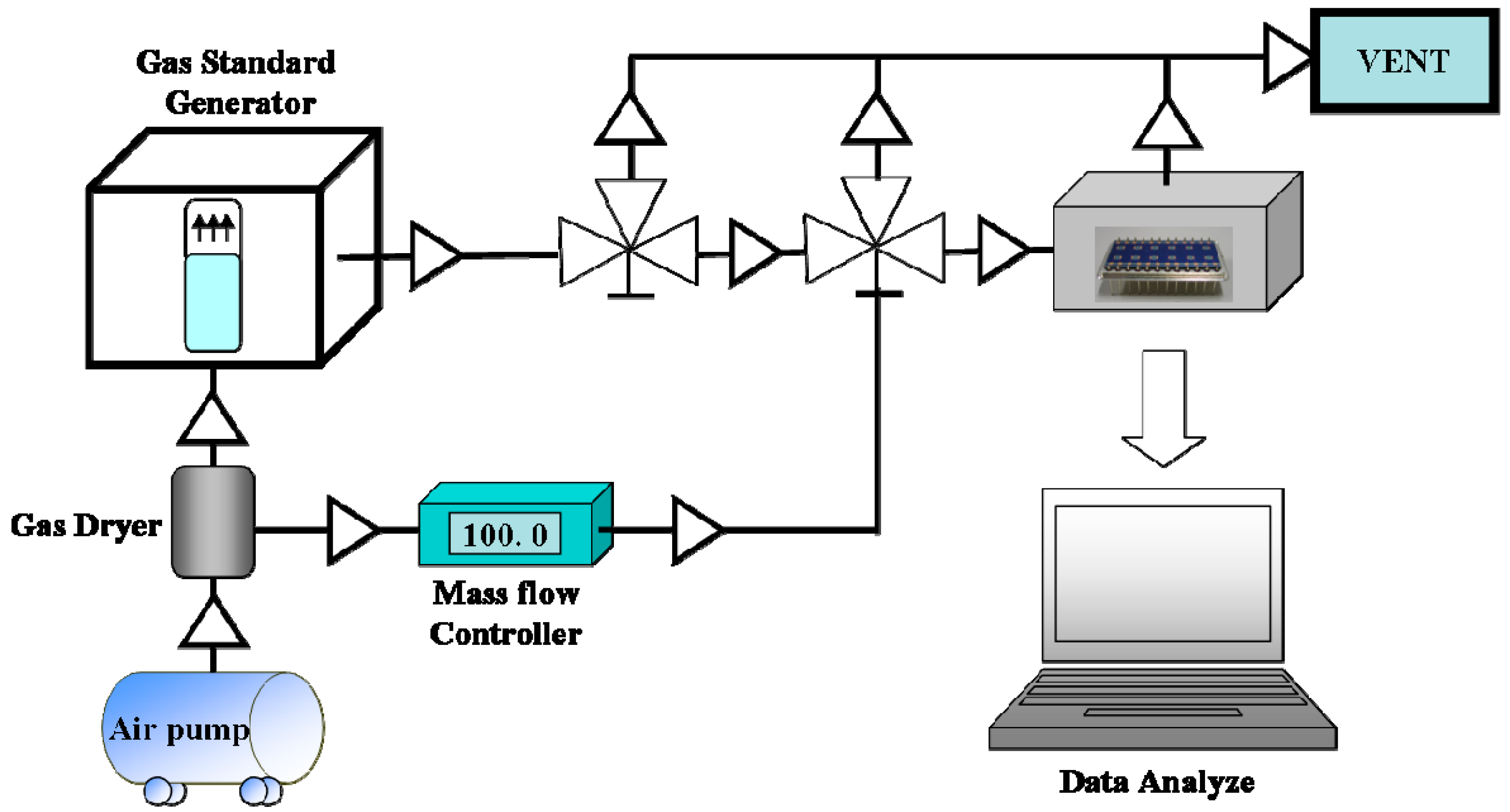
3. Results and Discussion
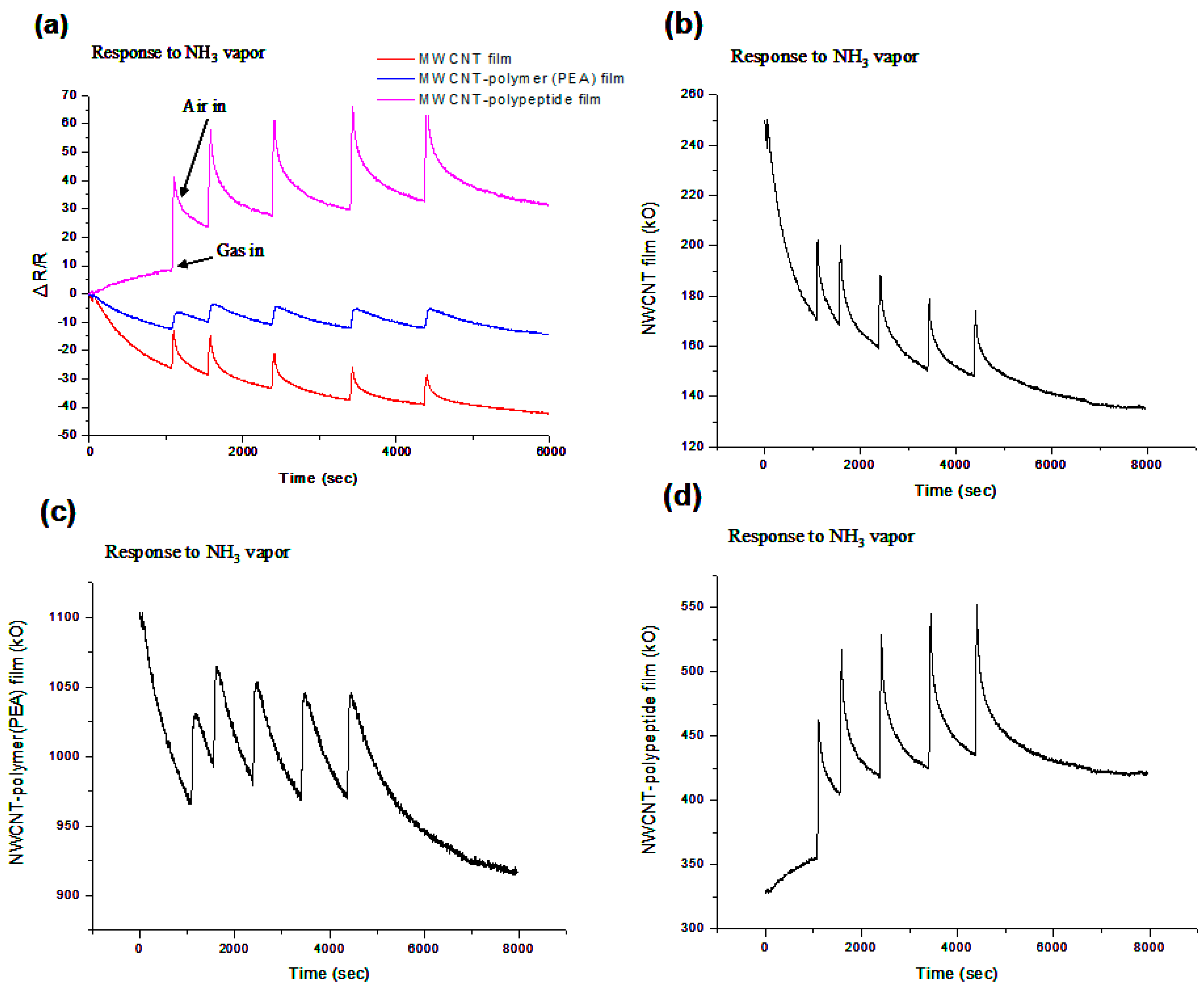
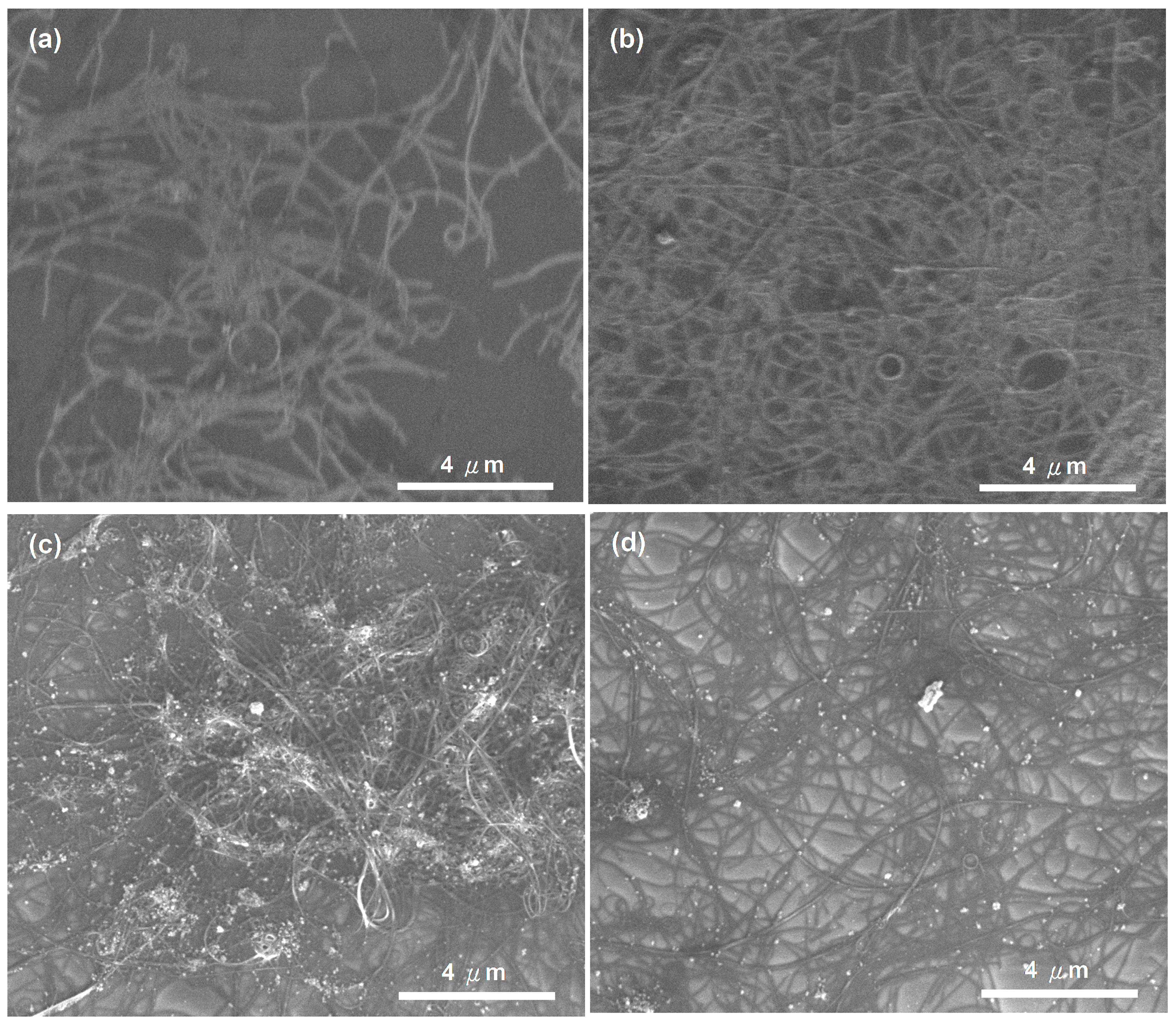
3.1. Performance of Three MWCNT-Assisted Sensing Membranes
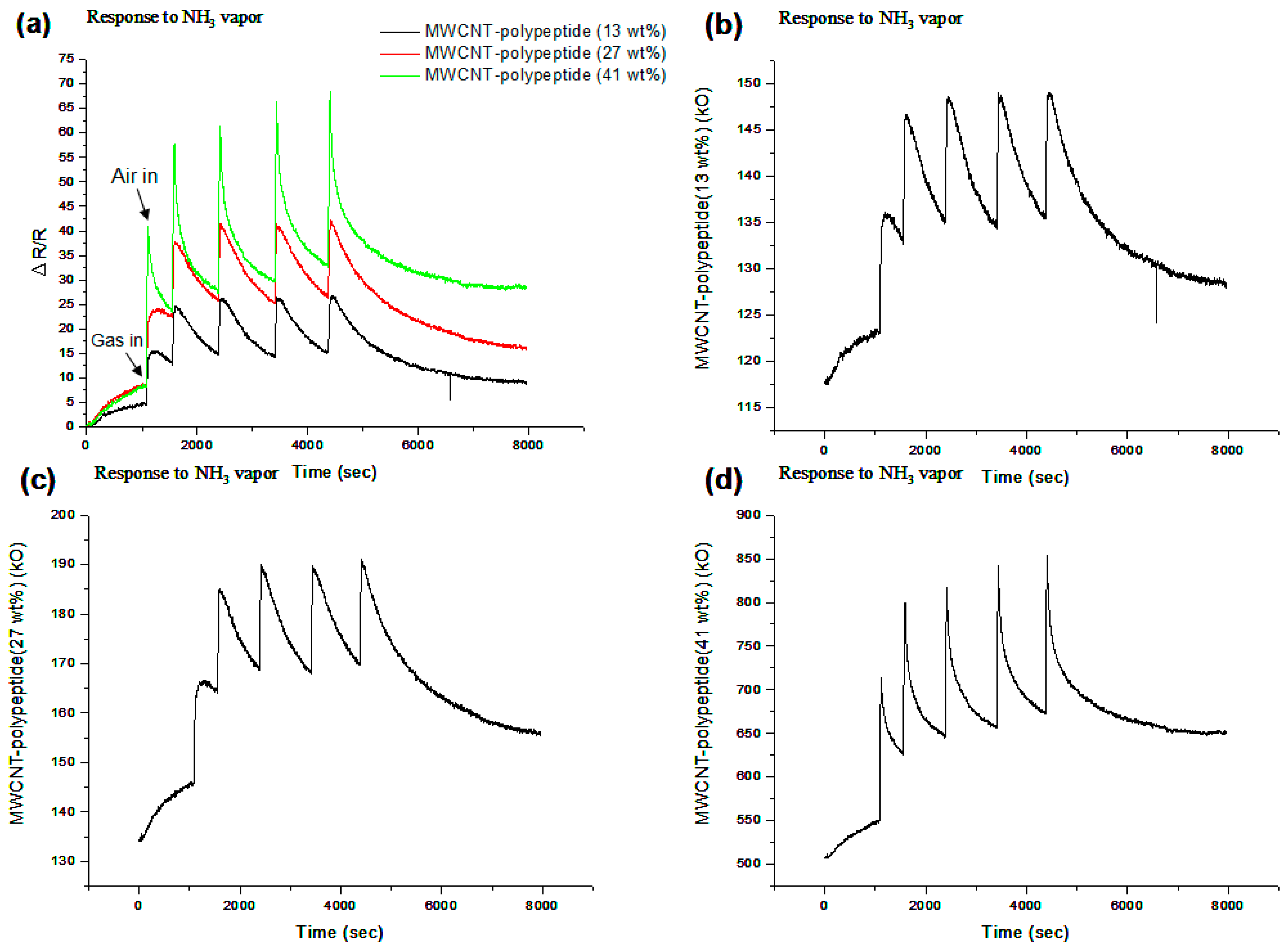
3.2. Effect of Polypeptide Ratios on Performance of MWNCT-Polypeptide Sensors
3.3. Sensor Device Response
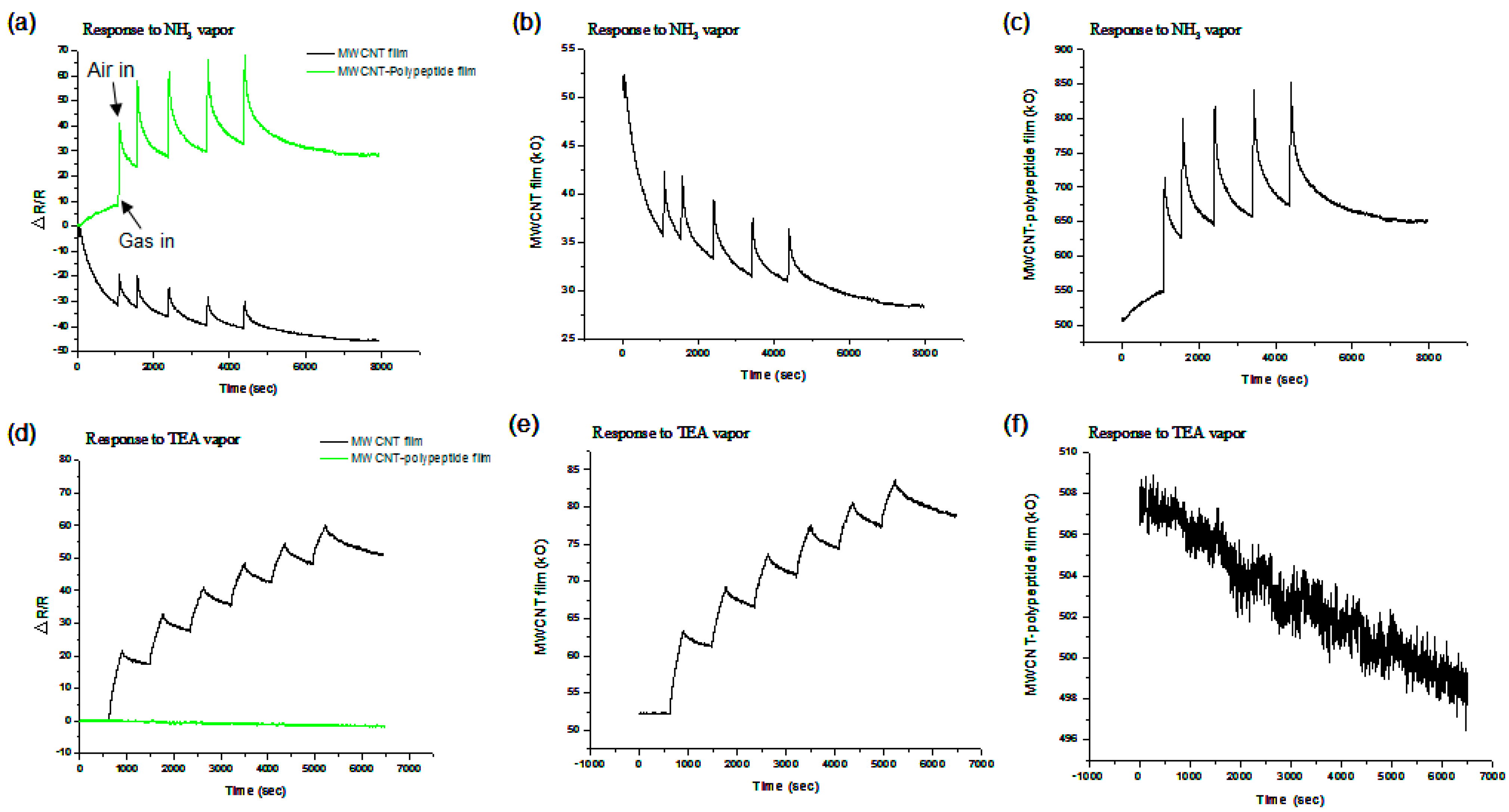
3.4. Potential for Long-Term Sensing
3.5. Discussion
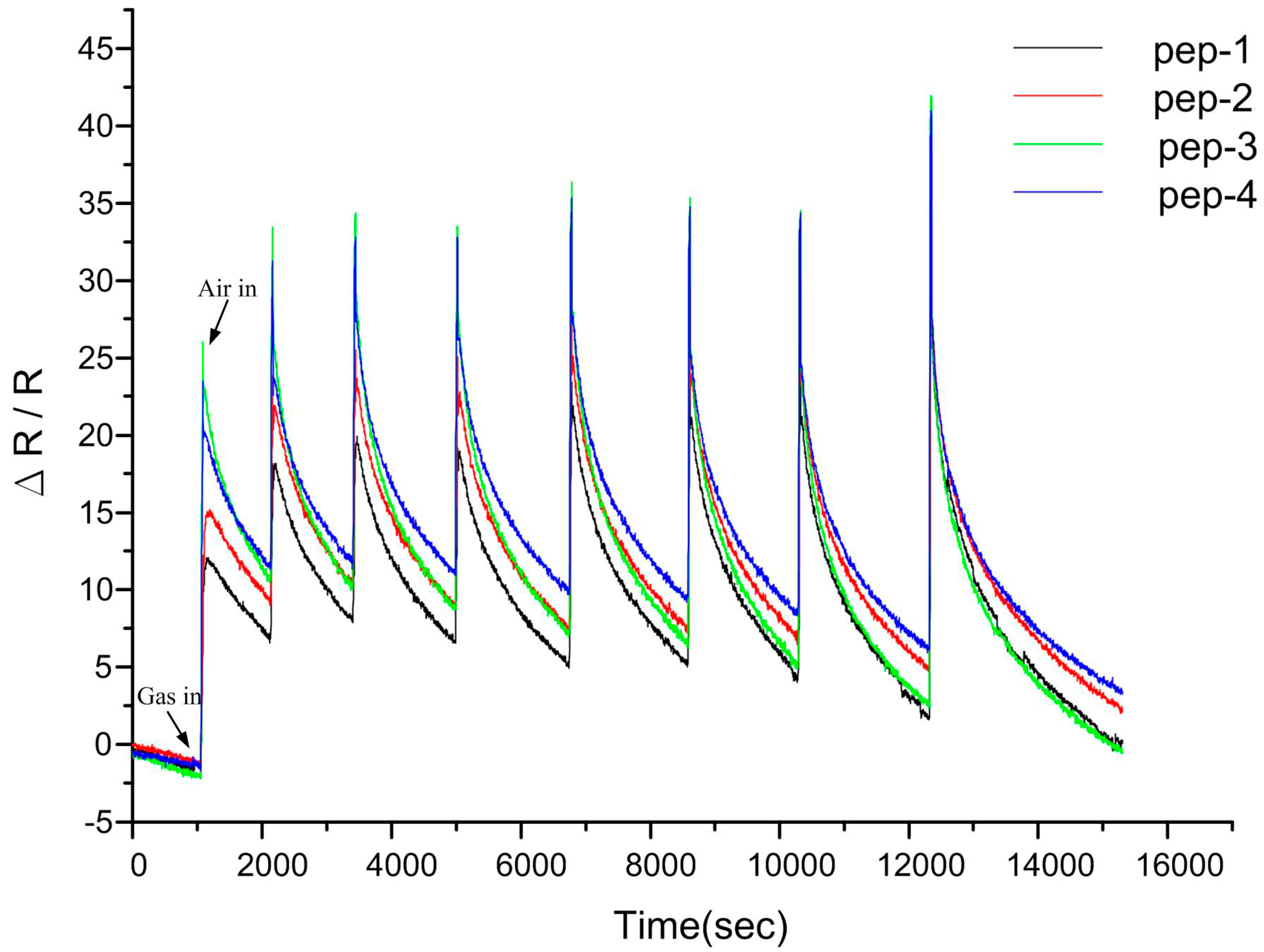
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bartlett, P.; Blair, N.; Gardner, J. Electronic Noses, Principles, Applications and Outlook. In Proceedings of the 15th Colloque A.S.I.C., Montpellier, France, 6—11 June 1993.
- Craven, M.; Gardner, J.; Bartlett, P. Electronic noses—Development and future prospects. TrAC Trends Anal. Chem. 1996, 15, 486–493. [Google Scholar]
- Kim, Y.S.; Ha, S.-C.; Yang, Y.; Kim, Y.J.; Cho, S.M.; Yang, H.; Kim, Y.T. Portable electronic nose system based on the carbon black-polymer composite sensor array. Sens. Actuators B Chem. 2005, 108, 285–291. [Google Scholar] [CrossRef]
- Lewis, N.S. Comparisons between mammalian and artificial olfaction based on arrays of carbon black-polymer composite vapor detectors. Acc. Chem. Res. 2004, 37, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Shevade, A.; Zhou, H.; Homer, M. Polymer-carbon black composite sensors in an electronic nose for air-quality monitoring. MRS Bull. 2004, 29, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.W. Review of conventional electronic noses and their possible application to the detection of explosives. In Electronic Noses & Sensors for the Detection of Explosives; Springer: Dordrecht, The Netherlands, 2004; pp. 1–28. [Google Scholar]
- Pearce, T.C.; Schiffman, S.S.; Nagle, H.T.; Gardner, J.W. Handbook of Machine Olfaction: Electronic Nose Technology; John Wiley & Sons: Weinheim, Germany, 2006. [Google Scholar]
- Göpel, W.; Schierbaum, K.D. SnO2 sensors: Current status and future prospects. Sens. Actuators B Chem. 1995, 26, 1–12. [Google Scholar] [CrossRef]
- Meixner, H.; Gerblinger, J.; Lampe, U.; Fleischer, M. Thin-film gas sensors based on semiconducting metal oxides. Sens. Actuators B Chem. 1995, 23, 119–125. [Google Scholar] [CrossRef]
- Slater, J.M.; Paynter, J.; Watt, E. Multi-layer conducting polymer gas sensor arrays for olfactory sensing. Analyst 1993, 118, 379–384. [Google Scholar] [CrossRef]
- Lonergan, M.C.; Severin, E.J.; Doleman, B.J.; Beaber, S.A.; Grubbs, R.H.; Lewis, N.S. Array-based vapor sensing using chemically sensitive, carbon black-polymer resistors. Chem. Mater. 1996, 8, 2298–2312. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. Application of conducting polymer technology in microsystems. Sens. Actuators A Phys. 1995, 51, 57–66. [Google Scholar] [CrossRef]
- Doleman, B.J.; Severin, E.J.; Lewis, N.S. Trends in odor intensity for human and electronic noses: Relative roles of odorant vapor pressure vs. molecularly specific odorant binding. Proc. Natl. Acad. Sci. USA 1998, 95, 5442–5447. [Google Scholar] [CrossRef] [PubMed]
- Severin, E.J.; Doleman, B.J.; Lewis, N.S. An investigation of the concentration dependence and response to analyte mixtures of carbon black/insulating organic polymer composite vapor detectors. Anal. Chem. 2000, 72, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.A.; Lewis, N.S. Low power, lightweight vapor sensing using arrays of conducting polymer composite chemically-sensitive resistors. Enantiomer 2000, 6, 159–170. [Google Scholar]
- Hagleitner, C.; Hierlemann, A.; Lange, D.; Kummer, A.; Kerness, N.; Brand, O.; Baltes, H. Smart single-chip gas sensor microsystem. Nature 2001, 414, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Liron, Z.; Kaushansky, N.; Frishman, G.; Kaplan, D.; Greenblatt, J. The polymer-coated SAW sensor as a gravimetric sensor. Anal. Chem. 1997, 69, 2848–2854. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Kadowaki, Y. Poly(acrylamide) derivatives for QCM-based HCl gas sensor applications. Sens. Actuators B Chem. 2008, 130, 842–847. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Uno, T. Molecular imprinting strategy for solvent molecules and its application for QCM-based VOC vapor sensing. Sens. Actuators B Chem. 2006, 113, 94–99. [Google Scholar] [CrossRef]
- Snow, E.; Perkins, F.; Houser, E.; Badescu, S.; Reinecke, T. Chemical detection with a single-walled carbon nanotube capacitor. Science 2005, 307, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Pham, A.; Gaillard, J.; Parker, A.; Rao, A. Carbon-nanotube-based resonant-circuit sensor for ammonia. Appl. Phys. Lett. 2002, 80, 4632–4634. [Google Scholar] [CrossRef]
- Someya, T.; Small, J.; Kim, P.; Nuckolls, C.; Yardley, J.T. Alcohol vapor sensors based on single-walled carbon nanotube field effect transistors. Nano Lett. 2003, 3, 877–881. [Google Scholar] [CrossRef]
- Snow, E.; Novak, J.; Campbell, P.; Park, D. Random networks of carbon nanotubes as an electronic material. Appl. Phys. Lett. 2003, 82, 2145–2147. [Google Scholar] [CrossRef]
- Suehiro, J.; Imakiire, H.; Hidaka, S.; Ding, W.; Zhou, G.; Imasaka, K.; Hara, M. Schottky-type response of carbon nanotube NO2 gas sensor fabricated onto aluminum electrodes by dielectrophoresis. Sens. Actuators B Chem. 2006, 114, 943–949. [Google Scholar] [CrossRef]
- Lucci, M.; Toschi, F.; Guglielmotti, V.; Orlanducci, S.; Terranova, M.L. Role of the Material Electrodes on Resistive Behaviour of Carbon Nanotube-Based Gas Sensors for H2S Detection. J. Sens. 2012, 2012. [Google Scholar] [CrossRef]
- Huan, T.N.; Ganesh, T.; Han, S.-H.; Yoon, M.-Y.; Chung, H. Sensitive detection of an anthrax biomarker using a glassy carbon electrode with a consecutively immobilized layer of polyaniline/carbon nanotube/peptide. Biosens. Bioelectron. 2011, 26, 4227–4230. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.R. Signaling pathways in odorant detection. Neuron 1992, 8, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Olfactory receptor based piezoelectric biosensors for detection of alcohols related to food safety applications. Sens. Actuators B Chem. 2011, 155, 8–18. [Google Scholar] [CrossRef]
- Chang, C.-P.; Chao, C.-Y.; Huang, J.H.; Li, A.-K.; Hsu, C.-S.; Lin, M.-S.; Hsieh, B.R.; Su, A.-C. Fluorescent conjugated polymer films as TNT chemosensors. Synth. Met. 2004, 144, 297–301. [Google Scholar] [CrossRef]
- Chang, C.-P.; Yuan, C.-L. The fabrication of a MWNTs–polymer composite chemoresistive sensor array to discriminate between chemical toxic agents. J. Mater. Sci. 2009, 44, 5485–5493. [Google Scholar] [CrossRef]
- Wang, L.; Tang, K.; Chiu, S.; Yang, S.; Kuo, C. A bio-inspired two-layer multiple-walled carbon nanotube-polymer composite sensor array and a bio-inspired fast-adaptive readout circuit for a portable electronic nose. Biosens. Bioelectron. 2011, 26, 4301–4307. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Tang, K.-T.; Teng, I.-J.; Kuo, C.-T.; Ho, C.-L.; Kuo, H.-W.; Su, T.-H.; Yang, S.-R.; Shi, G.-N.; Chang, C.-P. A single-walled carbon nanotube network gas sensing device. Sensors 2011, 11, 7763–7772. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Berger, F.; Picaud, F.; Ramseyer, C.; Glory, J.; Mayne-L’Hermite, M. Direct growth of the multi-walled carbon nanotubes as a tool to detect ammonia at room temperature. Chem. Phys. Lett. 2006, 433, 175–181. [Google Scholar] [CrossRef]
- Quang, N.H.; van Trinh, M.; Lee, B.-H.; Huh, J.-S. Effect of NH3 gas on the electrical properties of single-walled carbon nanotube bundles. Sens. Actuators B Chem. 2006, 113, 341–346. [Google Scholar] [CrossRef]
- Van Hieu, N.; Thuy, L.T.B.; Chien, N.D. Highly sensitive thin film NH3 gas sensor operating at room temperature based on SnO2/MWCNTs composite. Sens. Actuators B Chem. 2008, 129, 888–895. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-C.; Su, T.-H.; Ho, C.-L.; Yang, S.-R.; Chiu, S.-W.; Kuo, H.-W.; Tang, K.-T. A Bio-Inspired Two-Layer Sensing Structure of Polypeptide and Multiple-Walled Carbon Nanotube to Sense Small Molecular Gases. Sensors 2015, 15, 5390-5401. https://doi.org/10.3390/s150305390
Wang L-C, Su T-H, Ho C-L, Yang S-R, Chiu S-W, Kuo H-W, Tang K-T. A Bio-Inspired Two-Layer Sensing Structure of Polypeptide and Multiple-Walled Carbon Nanotube to Sense Small Molecular Gases. Sensors. 2015; 15(3):5390-5401. https://doi.org/10.3390/s150305390
Chicago/Turabian StyleWang, Li-Chun, Tseng-Hsiung Su, Cheng-Long Ho, Shang-Ren Yang, Shih-Wen Chiu, Han-Wen Kuo, and Kea-Tiong Tang. 2015. "A Bio-Inspired Two-Layer Sensing Structure of Polypeptide and Multiple-Walled Carbon Nanotube to Sense Small Molecular Gases" Sensors 15, no. 3: 5390-5401. https://doi.org/10.3390/s150305390





