Portable Nanoparticle-Based Sensors for Food Safety Assessment
Abstract
:1. Introduction
2. Common Types of Nanostructures in Nanotechnology-Based Sensing Approaches
2.1. Gold NPs
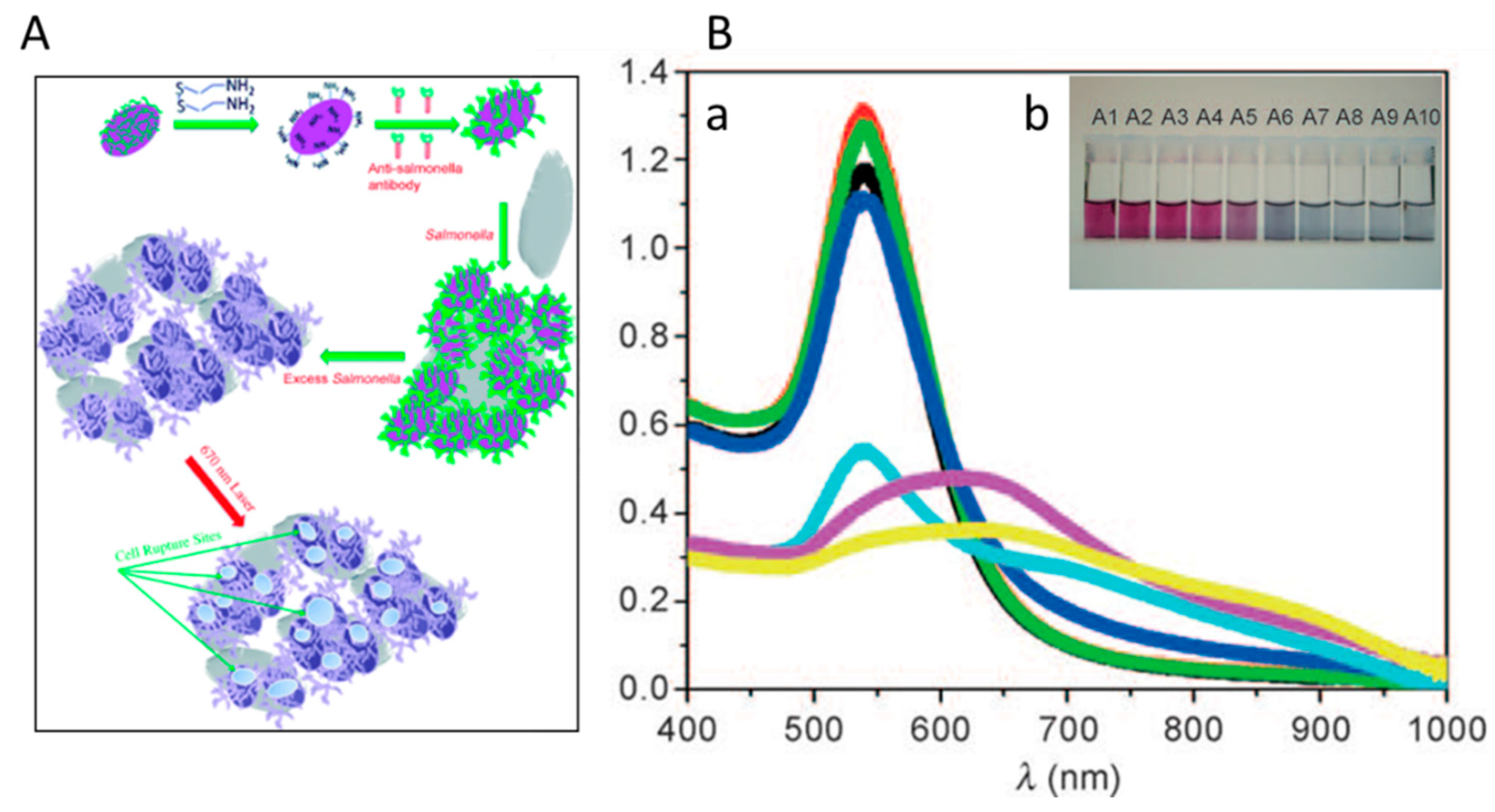
2.2. Silver NPs
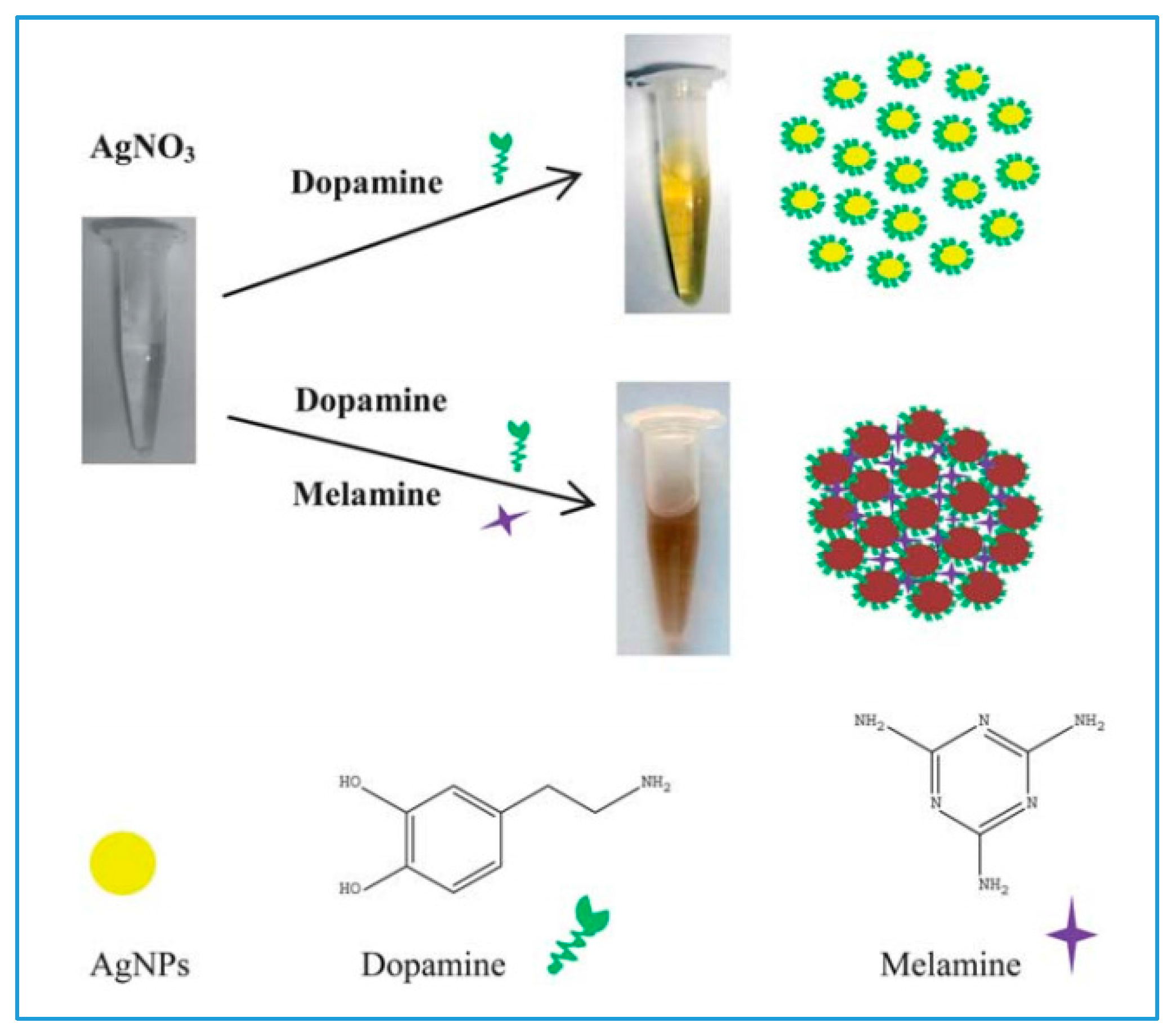
2.3. Cerium Oxide NPs
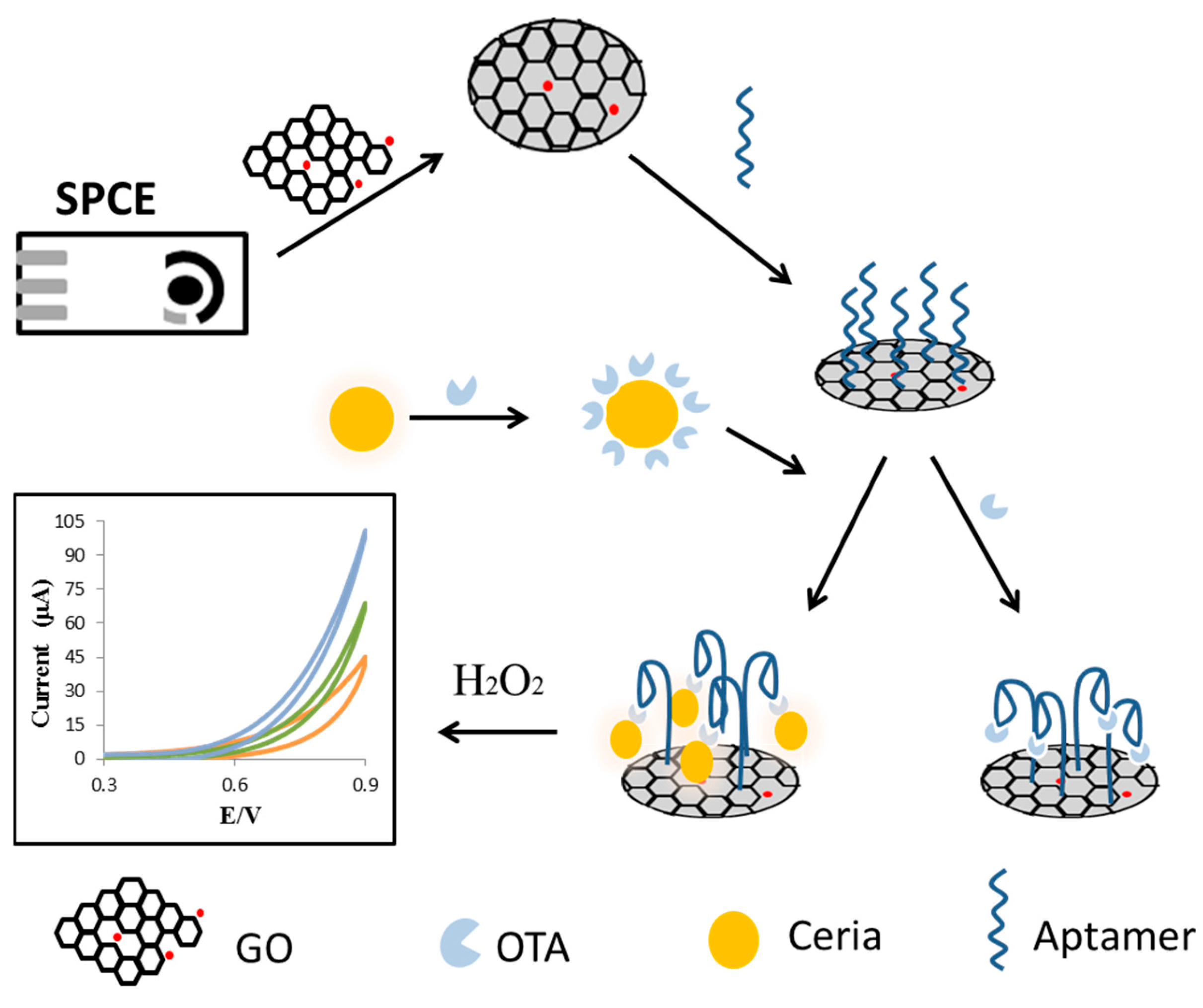
2.4. Carbon Nanotubes and Graphene
2.5. Magnetic Nanoparticles
2.6. Low-Cost Platforms for Portable NP-Based Detection
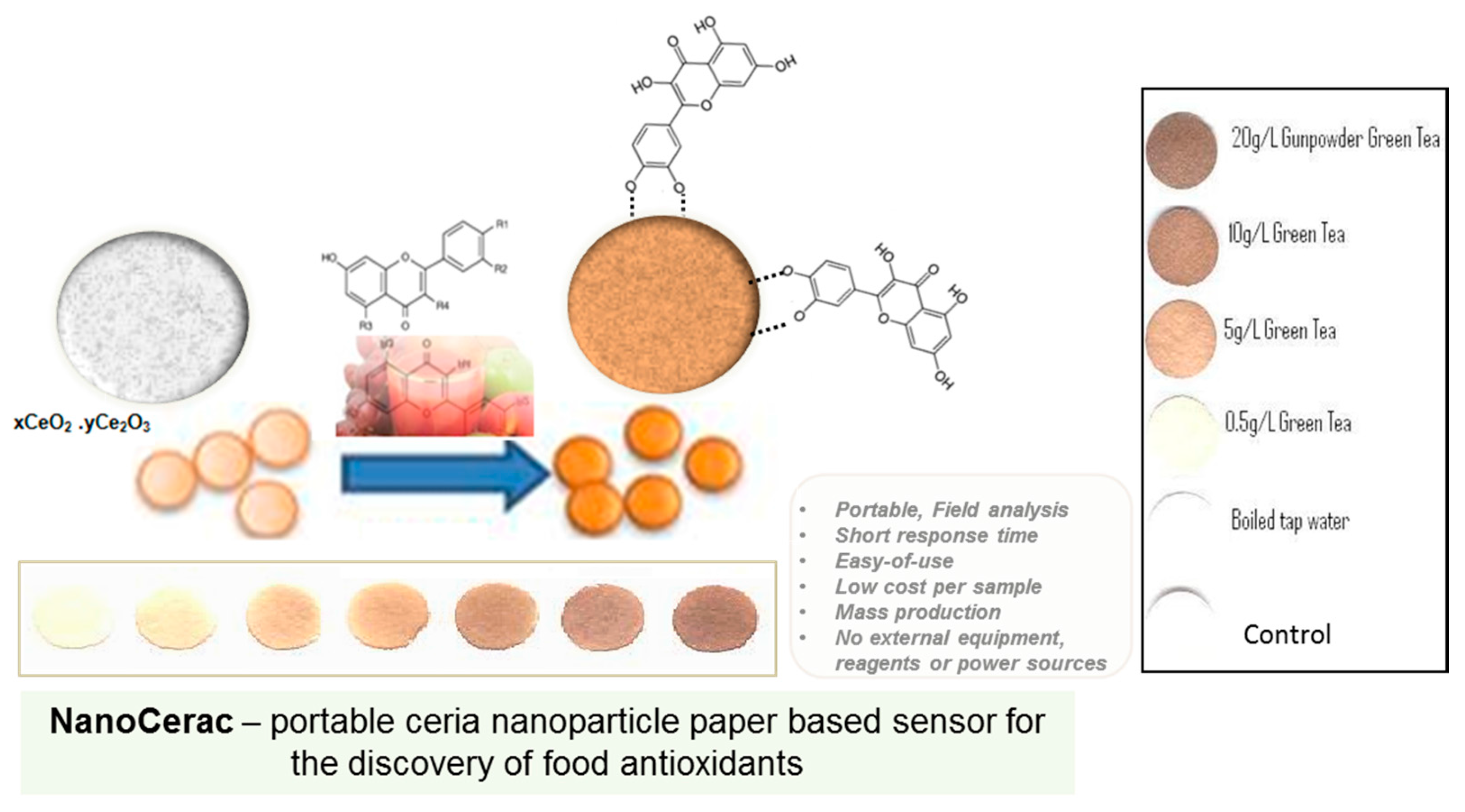
3. NP-Based Technologies for the Detection of Biological and Chemical Contaminants
3.1. Current Status of Foodborne-Related Illnesses
3.2. Detection of Microbial Contamination
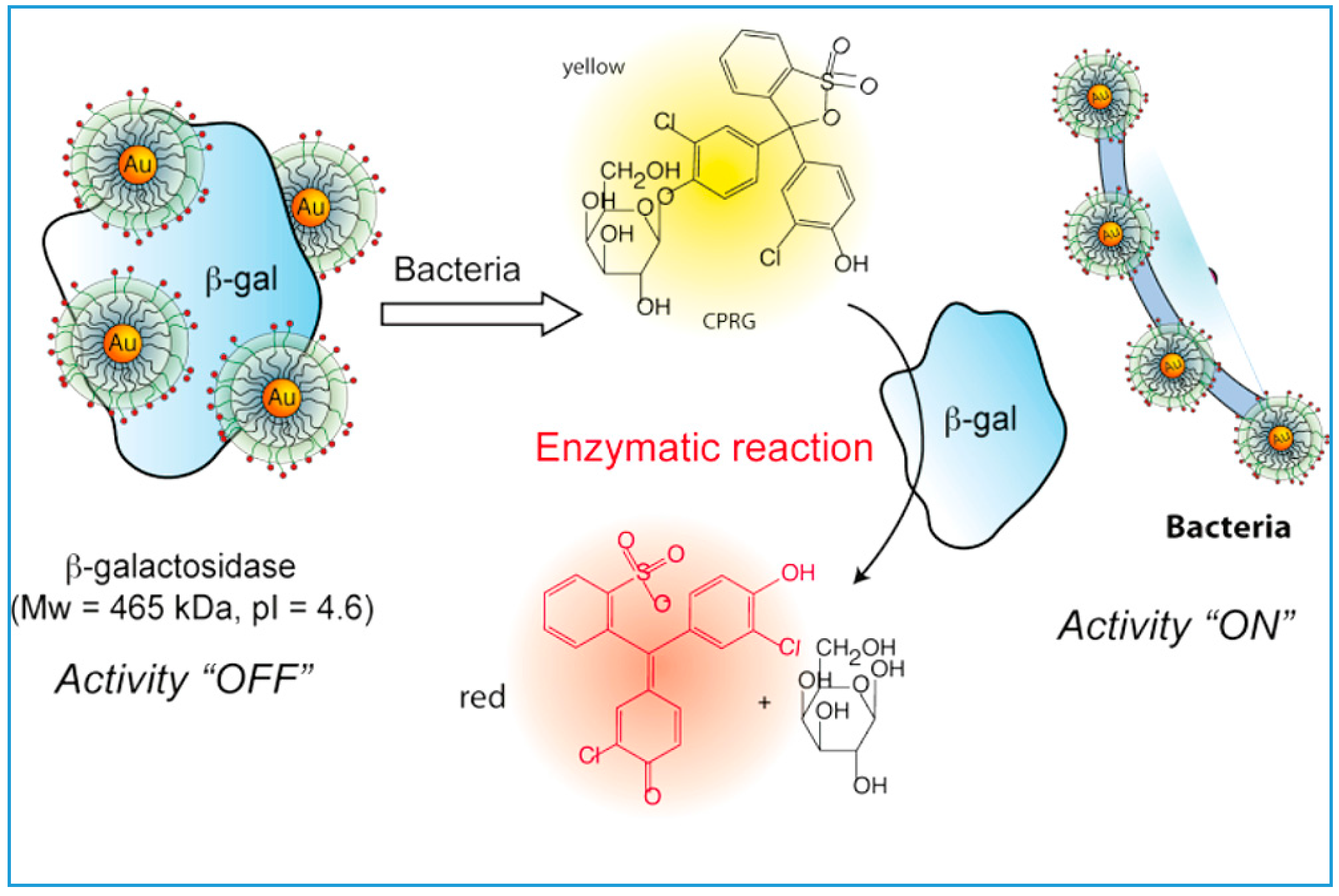


3.3. Detection of Pesticides
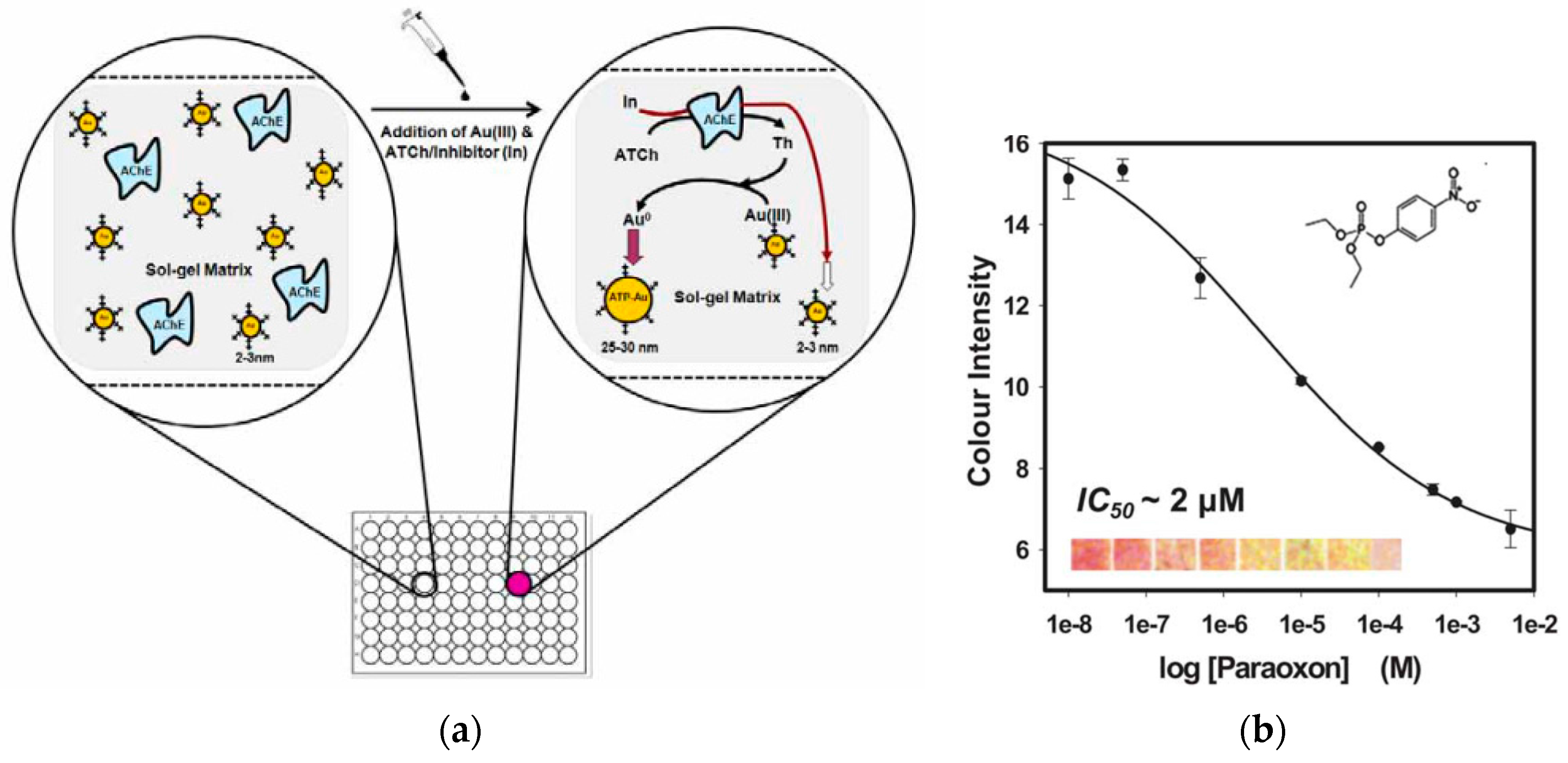
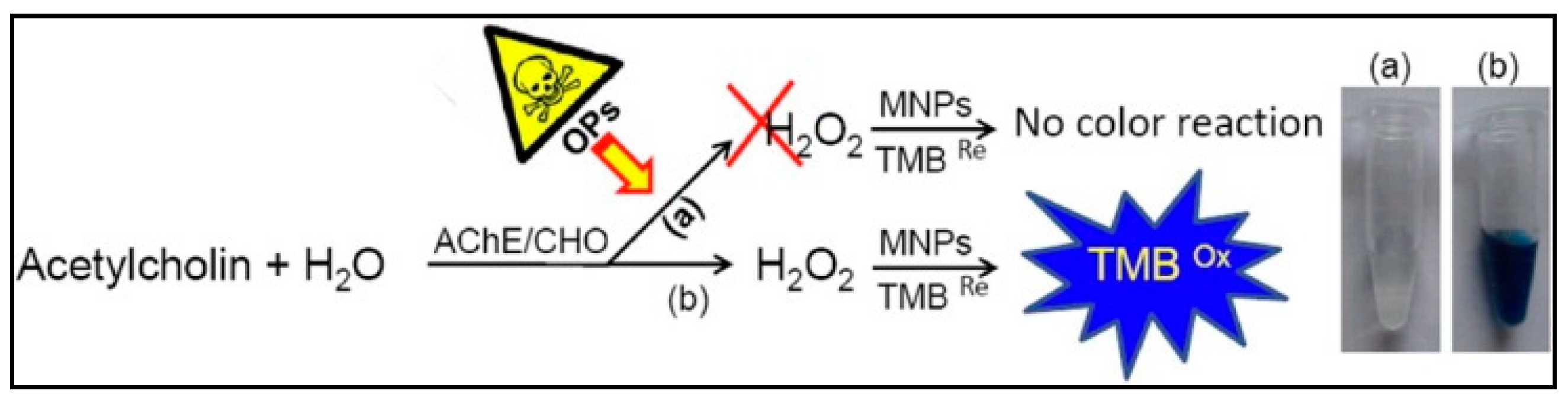
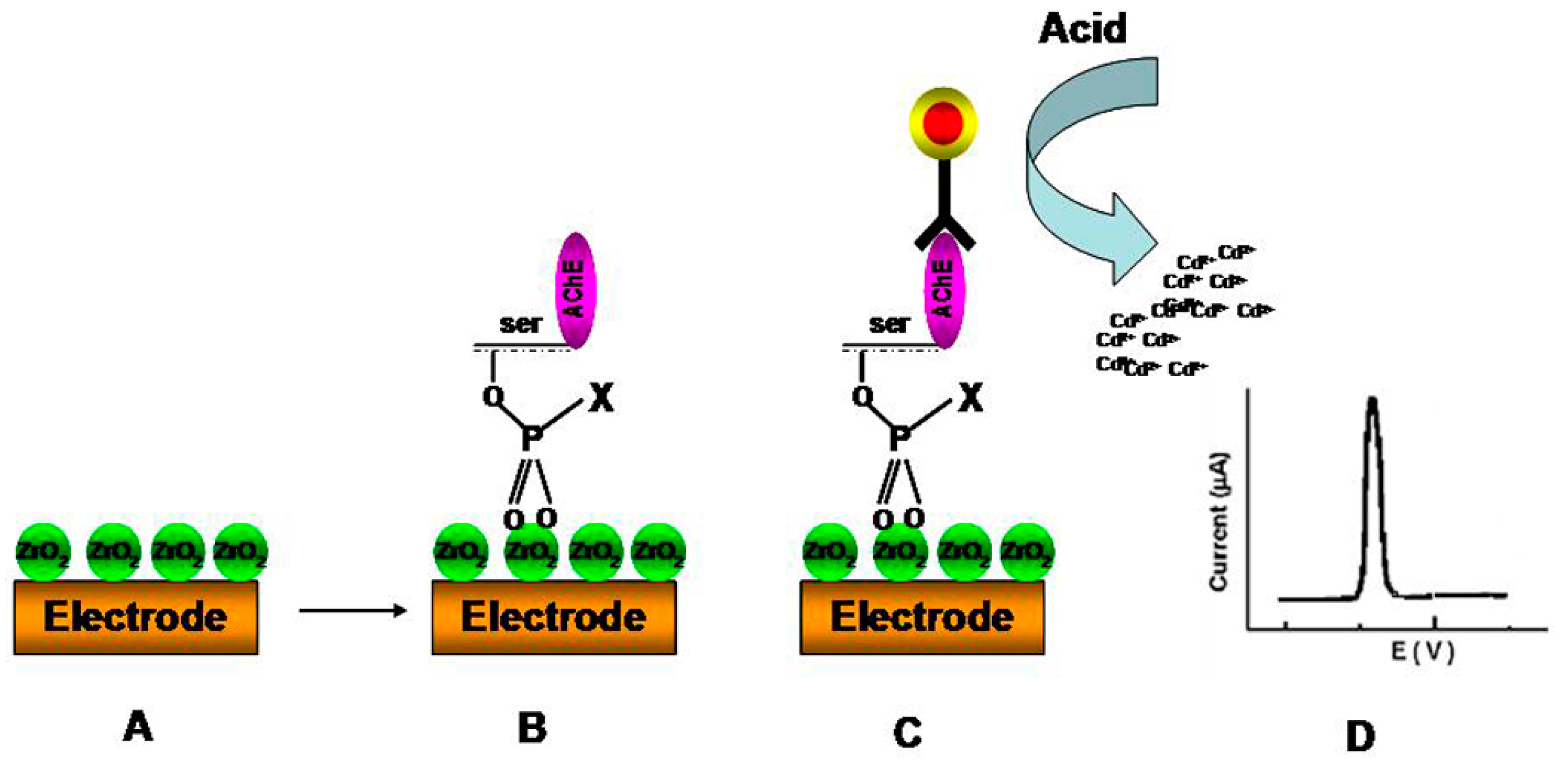
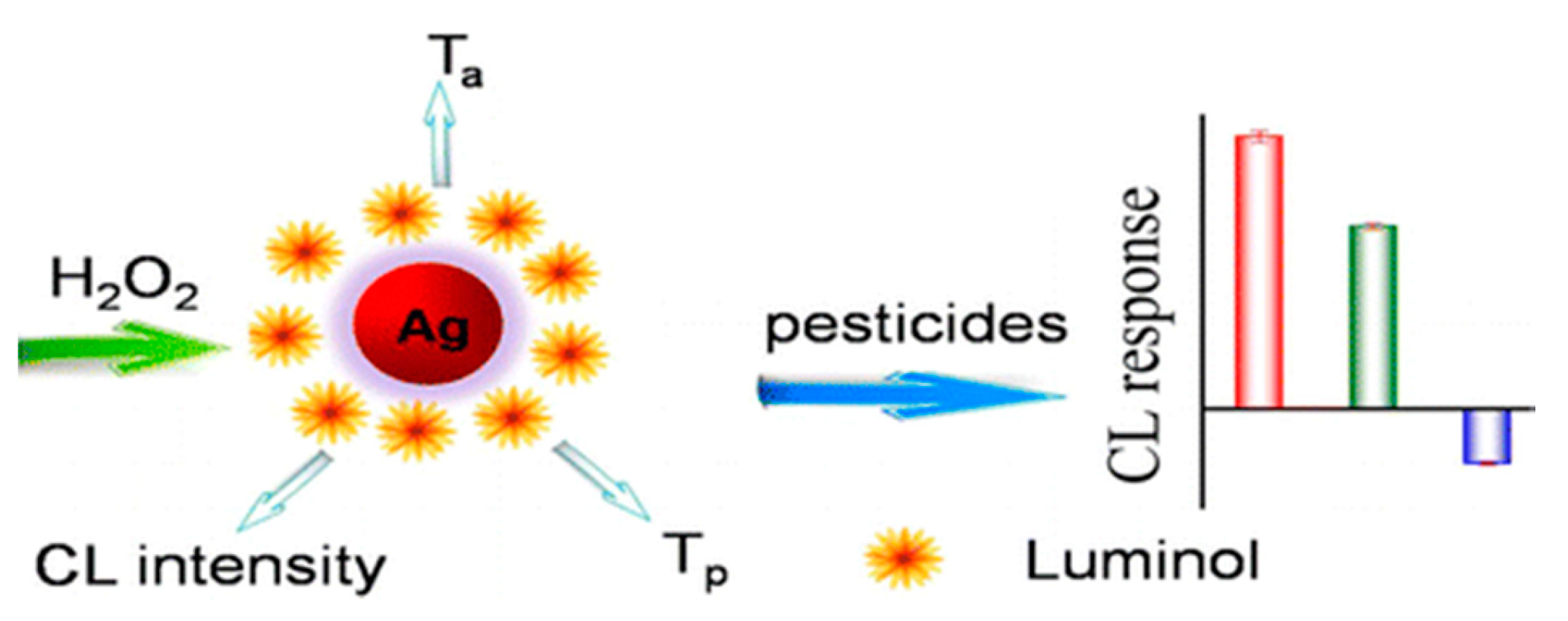
3.4. Detection of Metal Contaminants
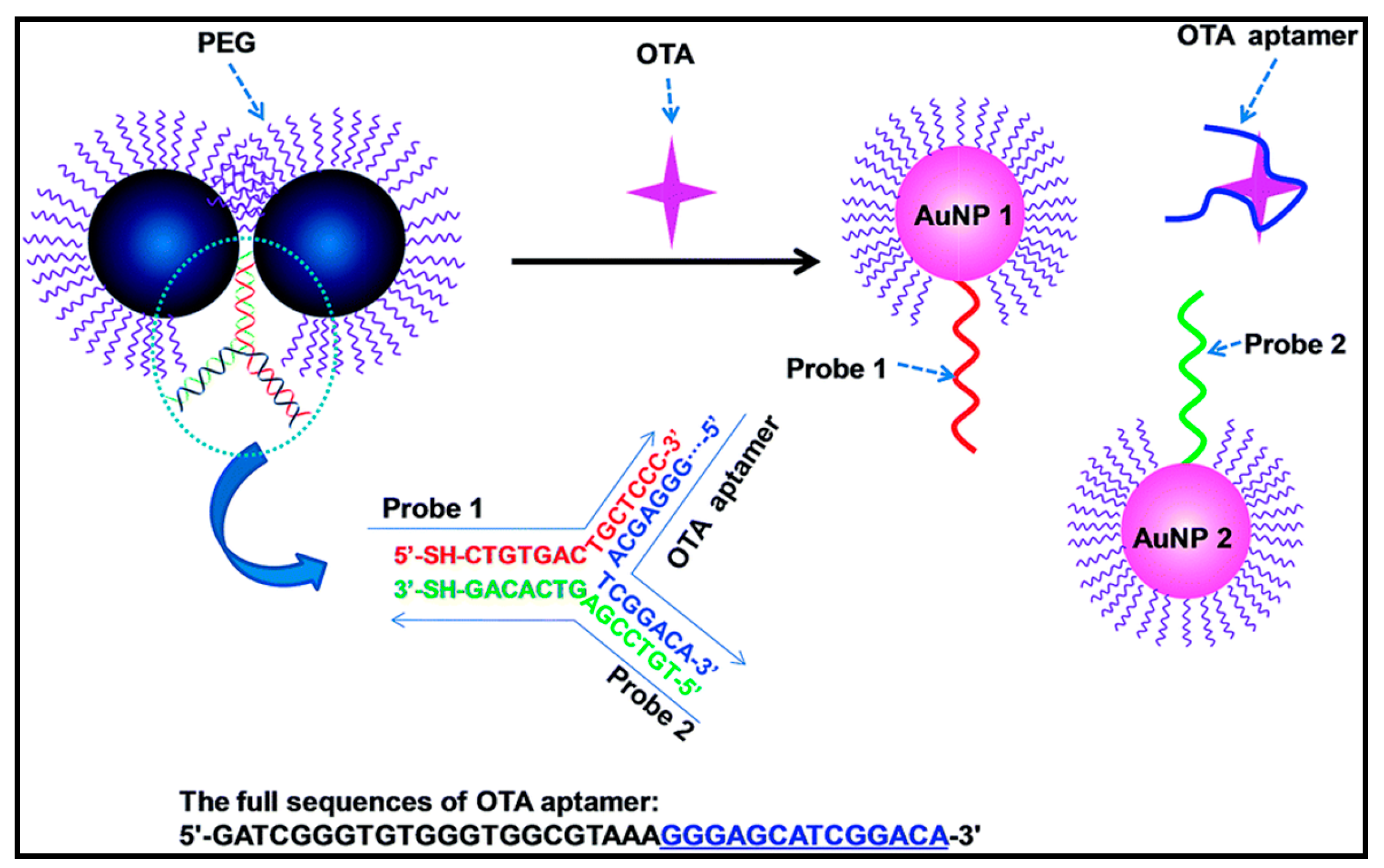
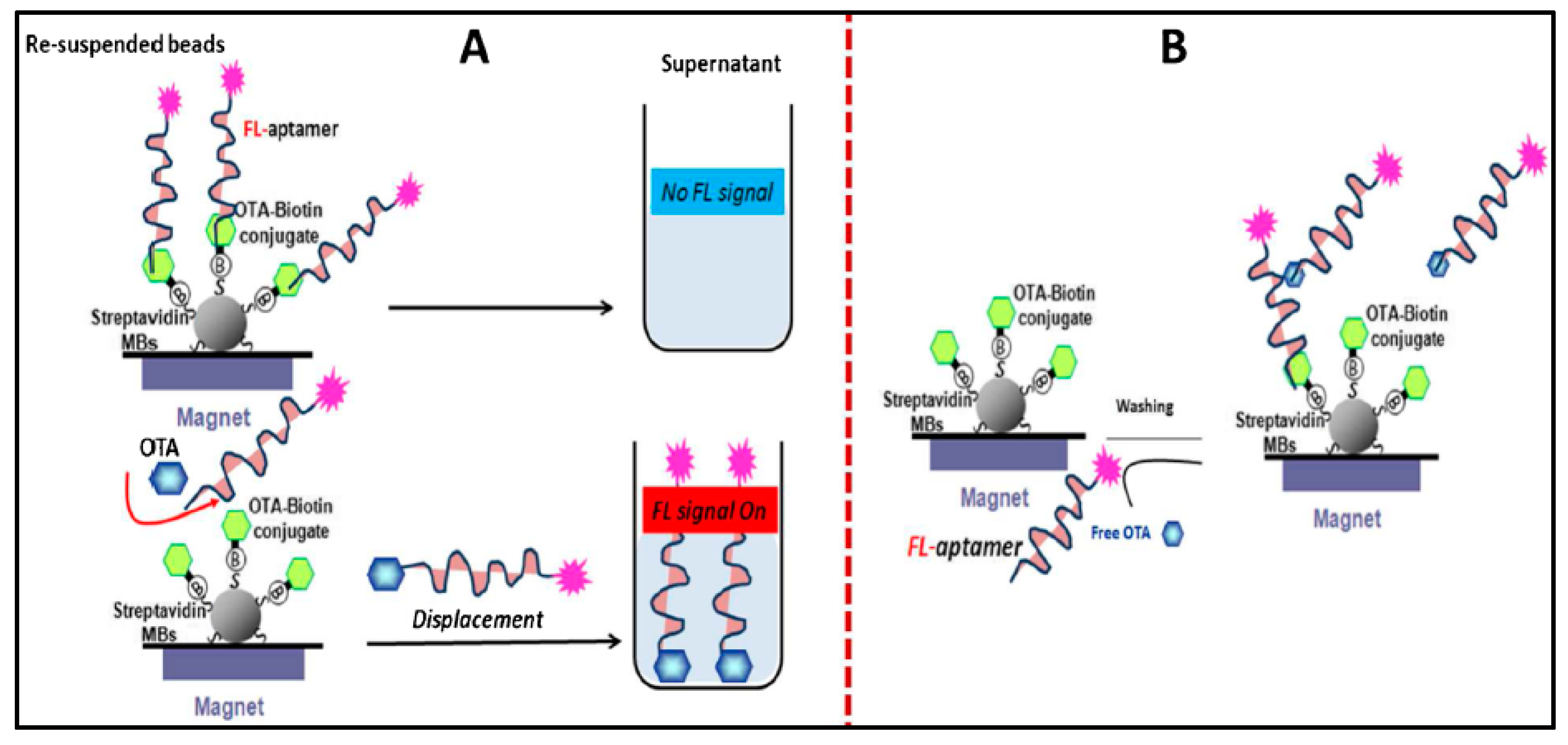
3.5. Detection of Mycotoxins
| Analyte | Assay Format | NPs Used | Detection Principle | Detection Limit | Dynamic Range | Refs. | |
|---|---|---|---|---|---|---|---|
| Microbial Contaminants | E. coli (XL1) | Colorimetric | AuNPs | Binding of bacteria to AuNPs leads the release of the enzyme attached on particles, restoring its activity | 1 × 102 bacteria/mL | 1 × 102–1 × 107 bacteria/mL | [40] |
| E. coli O157:H7 | Colorimetric | MNPs | The activity of intracellular enzymes measured | 5 cfu/mL | - | [101] | |
| E. coli BL 21 | 20 cfu/mL | ||||||
| E. coli O157:H7 | Colorimetric | AuNPs | Monoclonal antibody is conjugated to the colloidal AuNPs, and the sandwich format is utilized | 1.8 cfu/mL | 1.8 cfu–1.8 × 107 cfu/mL | [107] | |
| Salmonella | Colorimetric | MNPs and TiO2 nanocrystals | Salmonella were captured by antibody-immobilized magnetic nanoparticles and separated from solution, subsequent binding of antibody-conjugated TNs to the MNP–Salmonella complexes increased absorption | >100 cfu/mL | 108–102 cfu/mL | [114] | |
| E. coli | Colorimetric | MNPs | Amine-functionalized (AF) MNPs used for rapid capture and removal of bacterial pathogens by using plate counting method | - | - | [115] | |
| Sarcina lutea | |||||||
| Proteus vulgaris | |||||||
| Pesticides | Paraoxon | Colorimetric | AuNPs | Bioassay based on AChE-catalysed enlargement of AuNPs co-entrapped with the enzyme on paper. Hydrolysis of the enzyme substrate generated thiocholine, which further reduced the Au(III) to AuNPs, inducing particle growth and resulting in an increase in color intensity | 0.5 μM | 500 nM–1 mM | [125] |
| Sarin | Colorimetric | MNPs | ChO catalyzed the conversion of the product of the AChE reaction to hydrogen peroxide (H2O2). Produced H2O2 was then detected by the catalytic action of the MNPs on the oxidation of its substrate generating a color change. | 1 nM | - | [126] | |
| Methyl-paraoxon | 10 nM | ||||||
| Acephate | 5 μM | ||||||
| Phosphorylated AChE | Electrochemical | ZrO2 NPs | ZrO2NPs were used as sorbents for enzyme capture while quantum dots (QDs) were used as tags to label anti-AChE antibody and form a sandwich-like immunoreaction. The immunocaptured QD were determined by electrochemical stripping analysis of Cd ions after an acid-dissolution step of the QDs | 8.0 pM | 10 pM to 4 nM | [127] | |
| Dimethoate | Chemiluminescent | AgNPs | The array is based on the triple-channel properties of luminol-functionalized AgNPs and hydrogen peroxide chemiluminescent (CL) system containing CL intensity | 24 μg/mL | - | [128] | |
| Dipterex | |||||||
| Carbaryl | |||||||
| Chlorpyrifos | |||||||
| Carbofuran | |||||||
| Metals | Pb2+ | Colorimetric | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 400 μM | - | [35] |
| Hg2+ | Colorimetric | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 0.8 nM | 50–250 nM | [131] | |
| Hg2+ | Colorimetric | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 8 nM | 0.01–5 μM | [133] | |
| Hg2+ | Colorimetric | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 50 nM | 25–750 nM | [134] | |
| Hg2+ | Colorimetric | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 53 nM | 33–300 nM | [137] | |
| Pb2+ | 1 6 nM | 16 × 10−9 to 100 × 10−9 M | |||||
| Hg2+ | AgNPs | 16 nM | 16–660 nM | ||||
| Mn2+ | 16 × 10−9–50 × 10−8 M | ||||||
| Cd2+ | Colorimetric | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 16.6 nM | 0.5–16 μM | [138] | |
| Mycotoxins | Aflatoxin B1 | Colorimetric and Chemiluminescence | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 7 nM | 80–270 nM | [142] |
| Aflatoxin B2 | Colorimetric | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 25 pg/mL | 0.025–10 ng/mL | [143] | |
| Ochratoxin A | Colorimetric | AuNPs | Analyte induced aggregation/disaggregation phenomena of AuNPs | 0.05 nM | 0.2 nM to 250 nM | [144] | |
| Aflatoxin B1 (AFB) | Colorimetric | AuNPs | Competitive immunoassay between AFB modified magnetic beads and free AFB for AuNPs labelled antibodies. | 12 ng/L | 20–800 ng/L | [146] | |
| Ochratoxin A (OTA) | Fluorescent | Fluorescent particles | OTA-MBs (magnetic beads) were immobilized inside the wells and the analysis was performed by adding the fluorescent particles-modified aptamer which competed with the immobilized OTA and OTA in solution. The presence of OTA in solution prevented binding of the immobilized OTA to the aptamer, leading to a decrease of the fluorescence signal. | 0.005 nM | 0.1–150 nM | [149] |
4. Conclusions and Future Directions
Acknowledgments
Conflicts of Interest
References
- Yáñez, L.; Ortiz, D.; Calderón, J.; Batres, L.; Carrizales, L.; Mejía, J.; Martínez, L.; García-Nieto, E.; Díaz-Barriga, F. Overview of human health and chemical mixtures: Problems facing developing countries. Environ. Health Perspect. 2002, 110, 901–909. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC); 2010. Available online: http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on 5 November 2015).
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. “Plugging into enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xu, Y.; Zhu, N.N.; He, P.G.; Fang, Y.Z. An electrochemical DNA hybridization detection assay based on a silver nanoparticle label. Analyst 2002, 127, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhao, W.; Luo, X.L.; Chen, H.Y. A sensitive biosensor for lactate based on layer-by-layer assembling MnO2 nanoparticles and lactate oxidase on ion-sensitive field-effect transistors. Chem. Commun. 2005, 6, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, P.A.; Goncales, V.R.; Ponzio, E.A.; de Torresi, S.I.C. Synthesis, characterization and immobilization of Prussian blue nanoparticles. A potential tool for biosensing devices. Chem. Commun. 2005, 3, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Yuan, R.; Chai, Y.Q.; Tang, D.P.; Zhang, Y.; Wang, N.; Li, X.; Zhu, Q. A reagentless amperometric immunosensor based on gold nanoparticles/thionine/Nafion-membrane-modified gold electrode for determination of alpha-1-fetoprotein. Electrochem. Commun. 2005, 7, 355–360. [Google Scholar] [CrossRef]
- Wu, S.J.; Duan, N.; Ma, X.Y.; Xia, Y.; Wang, H.G.; Wang, Z.P.; Zhang, Q. Multiplexed Fluorescence Resonance Energy Transfer Aptasensor between Upconversion Nanoparticles and Graphene Oxide for the Simultaneous Determination of Mycotoxins. Anal. Chem. 2012, 84, 6263–6270. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Duato, P.; Castillo, J.R. An electrochemical immunosensor for ochratoxin A determination in wines based on a monoclonal antibody and paramagnetic microbeads. Anal. Bioanal. Chem. 2012, 403, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Bonel, L.; Vidal, J.C.; Duato, P.; Castillo, J.R. An electrochemical competitive biosensor for ochratoxin A based on a DNA biotinylated aptamer. Biosens. Bioelectron. 2011, 26, 3254–3259. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Marty, J.L.; Yang, X.R. Aptamer-based colorimetric biosensing of Ochratoxin A using unmodified gold nanoparticles indicator. Biosens. Bioelectron. 2011, 26, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Rae, A. Real Life Applications of Nanotechnology in Electronics. OnBoard Technol. 2006, 2006, 28. [Google Scholar]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Coll. Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Reviews 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Sozer, N.; Kokini, J.L. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009, 27, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chellaram, C.; Murugaboopathi, G.; John, A.A.; Sivakumar, R.; Ganesan, S.; Krithika, S.; Priya, G. Significance of Nanotechnology in Food Industry. APCBEE Proced. 2014, 8, 109–113. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Mirica, K.A.; Dasgupta, R.; Dickey, M.D.; Butte, M.J.; Whitesides, G.M. Thread as a Matrix for Biomedical Assays. ACS Appl. Mater. Interfaces 2010, 2, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2009, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ali, M.M.; Aguirre, S.D.; Brook, M.A.; Li, Y. Paper-Based Bioassays Using Gold Nanoparticle Colorimetric Probes. Anal. Chem. 2008, 80, 8431–8437. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, H.; Vongsvivut, J.; Li, R.; Glushenkov, A.M.; He, J.; Chen, Y.; Barrow, C.J.; Yang, W. Self-assembly of core-satellite gold nanoparticles for colorimetric detection of copper ions. Anal. Chim. Acta 2013, 803, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, H.; He, Y.; Ding, N.; Cao, Q. Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles. Coll. Surf. A 2011, 391, 179–183. [Google Scholar] [CrossRef]
- Sherry, L.J.; Jin, R.; Mirkin, C.A.; Schatz, G.C.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy of Single Silver Triangular Nanoprisms. Nano Lett. 2006, 6, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.W.; Jin, A.R.; Mirkin, C.A. DNA-Modified Core-Shell Ag/Au Nanoparticles. J. Am. Chem. Soc. 2001, 123, 7961–7962. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, C.; Han, B.; Wang, E. Enzyme Colorimetric Assay Using Unmodified Silver Nanoparticles. Anal. Chem. 2008, 80, 7051–7055. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Storhoff, J.J.; Lazarides, A.A.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L.; Schatz, G.C. What Controls the Optical Properties of DNA-Linked Gold Nanoparticle Assemblies? J. Am. Chem. Soc. 2000, 122, 4640–4650. [Google Scholar] [CrossRef]
- Liu, M.; Jia, C.; Jin, Q.; Lou, X.; Yao, S.; Xiang, J.; Zhao, J. Novel colorimetric enzyme immunoassay for the detection of carcinoembryonic antigen. Talanta 2010, 81, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Airò, F.; Merkoçi, A. Enhanced Gold Nanoparticle Based ELISA for a Breast Cancer Biomarker. Anal. Chem. 2010, 82, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Liu, S.-W.; Lin, C.-M.; Chen, C.-H. Recognition of Potassium Ion in Water by 15-Crown-5 Functionalized Gold Nanoparticles. Anal. Chem. 2002, 74, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Chen, C.-H.; Lin, M.-C.; Hsu, H.-F. A Cooperative Effect of Bifunctionalized Nanoparticles on Recognition: Sensing Alkali Ions by Crown and Carboxylate Moieties in Aqueous Media. Anal. Chem. 2005, 77, 4821–4828. [Google Scholar] [CrossRef] [PubMed]
- Obare, S.O.; Hollowell, R.E.; Murphy, C.J. Sensing Strategy for Lithium Ion Based on Gold Nanoparticles. Langmuir 2002, 18, 10407–10410. [Google Scholar] [CrossRef]
- Kim, Y.; Johnson, R.C.; Hupp, J.T. Gold Nanoparticle-Based Sensing of “Spectroscopically Silent” Heavy Metal Ions. Nano Lett. 2001, 1, 165–167. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wu, S.H.; Chen, C. A simple strategy for prompt visual sensing by gold nanoparticles: General applications of interparticle hydrogen bonds. Angew. Chem. Int. Ed. 2006, 45, 4948–4951. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Han, M.S.; Mirkin, C.A. Colorimetric Detection of Mercuric Ion (Hg2+) in Aqueous Media using DNA-Functionalized Gold Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, J.H.; Lu, Y. Label-Free Colorimetric Detection of Lead Ions with a Nanomolar Detection Limit and Tunable Dynamic Range by using Gold Nanoparticles and DNAzyme. Adv. Mater. 2008, 20, 3263–3267. [Google Scholar] [CrossRef]
- Phillips, R.L.; Miranda, O.R.; You, C.C.; Rotello, V.M.; Bunz, U.H. Rapid and Efficient Identification of Bacteria Using Gold-Nanoparticle–Poly (para-phenyleneethynylene) Constructs. Angew. Chem. Int. Ed. 2008, 47, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Miranda, O.R.; Li, X.; Garcia-Gonzalez, L.; Zhu, Z.-J.; Yan, B.; Bunz, U.H.; Rotello, V.M. Colorimetric Bacteria Sensing Using a Supramolecular Enzyme-Nanoparticle Biosensor. J. Am. Chem. Soc. 2011, 133, 9650–9653. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Singh, A.K.; Senapati, D.; Neely, A.; Yu, H.T.; Ray, P.C. Rapid Colorimetric Identification and Targeted Photothermal Lysis of Salmonella Bacteria by Using Bioconjugated Oval-Shaped Gold Nanoparticles. Chem. Eur. J. 2010, 16, 5600–5606. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jin, R.; Mirkin, C.A. DNA-modified core-shell Ag/Au nanoparticles. J. Am. Chem. Soc. 2001, 123, 7961–7962. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Yoosaf, K.; Ipe, B.I.; Suresh, C.H.; Thomas, K.G. In Situ Synthesis of Metal Nanoparticles and Selective Naked-Eye Detection of Lead Ions from Aqueous Media. J. Phys. Chem. C 2007, 111, 12839–12847. [Google Scholar] [CrossRef]
- Schofield, C.L.; Haines, A.H.; Field, R.A.; Russell, D.A. Silver and Gold Glyconanoparticles for Colorimetric Bioassays. Langmuir 2006, 22, 6707–6711. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, F.; Wan, Y.; Ma, L. Colorimetric detection of melamine in pretreated milk using silver nanoparticles functionalized with sulfanilic acid. Food Control 2015, 50, 356–361. [Google Scholar] [CrossRef]
- Xiong, D.; Li, H. Colorimetric detection of pesticides based on calixarene modified silver nanoparticles in water. Nanotechnology 2008, 19, 465502. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Niu, H.; Zhang, X.; Cai, Y. One-step synthesis of silver/dopamine nanoparticles and visual detection of melamine in raw milk. Analyst 2011, 136, 4192–4196. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Lytton-Jean, A.K.; Hurst, S.J.; Mirkin, C.A. Silver nanoparticle-oligonucleotide conjugates based on DNA with triple cyclic disulfide moieties. Nano Lett. 2007, 7, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, G.; Hayat, A.; Andreescu, S. A generic amplification strategy for electrochemical aptasensors using a non-enzymatic nanoceria tag. Nanoscale 2015, 7, 13230–12338. [Google Scholar] [CrossRef] [PubMed]
- Ornatska, M.; Sharpe, E.; Andreescu, D.; Andreescu, S. Paper Bioassay Based on Ceria Nanoparticles as Colorimetric Probes. Anal. Chem. 2011, 83, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Andreescu, S. Nanoceria Particles As Catalytic Amplifiers for Alkaline Phosphatase Assays. Anal. Chem. 2013, 85, 10028–10032. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Bulbul, G.; Andreescu, S. Probing phosphatase activity using redox active nanoparticles: A novel colorimetric approach for the detection of enzyme activity. Biosens. Bioelectron. 2014, 56, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-Like Activity of Polymer-Coated Cerium Oxide Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Frasco, T.; Andreescu, D.; Andreescu, S. Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac). Analyst 2013, 138, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Evers, T.H.; Prins, M.W.J. How Antibody Surface Coverage on Nanoparticles Determines the Activity and Kinetics of Antigen Capturing for Biosensing. Anal. Chem. 2014, 86, 8158–8166. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Bradley, R.; Frasco, T.; Jayathilaka, D.; Marsh, A.; Andreescu, S. Metal oxide based multisensor array and portable database for field analysis of antioxidants. Sens. Actuat. B Chem. 2014, 193, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Small, J.P.; Amori, M.E.S.; Kim, P. Electric Field Modulation of Galvanomagnetic Properties of Mesoscopic Graphite. Phys. Rev. Lett. 2005, 94, 176803. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, Y.; Lu, Y.; Kybert, N.J.; Luo, Z.; Johnson, A.T.C. Intrinsic Response of Graphene Vapor Sensors. Nano Lett. 2009, 9, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bo, Z.; Lu, G. Vertically-Oriented Graphene for Sensing and EnvironmentalApplications. In Vertically-Oriented Graphene; Springer International Publishing: Cham, Switzerland, 2015; pp. 67–77. [Google Scholar]
- Huang, L.; Wu, J.; Zheng, L.; Qian, H.; Xue, F.; Wu, Y.; Pan, D.; Adeloju, S.B.; Chen, W. Rolling Chain Amplification Based Signal-Enhanced Electrochemical Aptasensor for Ultrasensitive Detection of Ochratoxin A. Anal. Chem. 2013, 85, 10842–10849. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Gu, Y.; Lan, H.; Sun, Y.; Gao, H. Functional graphene-gold nano-composite fabricated electrochemical biosensor for direct and rapid detection of bisphenol, A. Anal. Chim. Acta 2015, 853, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, J.; Li, D.; Li, Y. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Pan, J.; Pan, K.; Yu, Y.; Zhong, A.; Wei, S.; Li, J.; Shi, J.; Li, X. An electrochemical sensor for hydrazine and nitrite based on graphene–cobalt hexacyanoferrate nanocomposite: Toward environment and food detection. J. Electroanal. Chem. 2015, 745, 80–87. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Du, D.; Lin, Y. Acetylcholinesterase biosensor based on a gold nanoparticle-polypyrrole-reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst 2014, 139, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Lin, Z.; Zhang, D.; Wang, Y.; Hou, B. Impedimetric immunosensor doped with reduced graphene sheets fabricated by controllable electrodeposition for the non-labelled detection of bacteria. Biosens. Bioelectron. 2011, 26, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Huang, N.M.; Lim, H.N.; Zainy, M.; Harrison, I.; Chia, C.H. Preparation of highly water dispersible functional graphene/silver nanocomposite for the detection of melamine. Sens. Actuat. B Chem. 2013, 181, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Sheng, L.; Ren, J.; Miao, Y.; Wang, J.; Wang, E. PVP-coated graphene oxide for selective determination of ochratoxin A via quenching fluorescence of free aptamer. Biosens. Bioelectron. 2011, 26, 3494–3499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, P.; Ye, B.-C. A low-cost and simple paper-based microfluidic device for simultaneous multiplex determination of different types of chemical contaminants in food. Biosens. Bioelectron. 2015, 68, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; de Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Yang, C.; Rhouati, A.; Marty, J.L. Recent advances and achievements in nanomaterial-based, and structure switchable aptasensing platforms for ochratoxin A detection. Sensors 2013, 13, 15187–15208. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Catanante, G.; Marty, J.L. Current Trends in Nanomaterial-Based Amperometric Biosensors. Sensors 2014, 14, 23439–23461. [Google Scholar] [CrossRef] [PubMed]
- Van Dorst, B.; Mehta, J.; Bekaert, K.; Rouah-Martin, E.; de Coen, W.; Dubruel, P.; Blust, R.; Robbens, J. Recent advances in recognition elements of food and environmental biosensors: A review. Biosens. Bioelectron. 2010, 26, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Taitt, C.; Ligler, F.; Anderson, G. Multiplexed magnetic microsphere immunoassays for detection of pathogens in foods. Sens. Instrum. Food Qual. Saf. 2010, 4, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Alcaine, S.D.; Jiang, Z.; Rotello, V.M.; Nugen, S.R. Detection of Escherichia coli in Drinking Water Using T7 Bacteriophage-Conjugated Magnetic Probe. Anal. Chem. 2015, 87, 8977–8984. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.B.; Alonso, G.A.; Muñoz, R.; Hayat, A.; Marty, J.-L. Design of a novel magnetic particles based electrochemical biosensor for organophosphate insecticide detection in flow injection analysis. Sens. Actuat. B Chem. 2015, 208, 491–496. [Google Scholar] [CrossRef]
- Jubete, E.; Loaiza, O.A.; Ochoteco, E.; Pomposo, J.A.; Grande, H.; Rodriguez, J. Nanotechnology: A Tool for Improved Performance on Electrochemical Screen-Printed (Bio)Sensors. J. Sensors 2009, 2009. [Google Scholar] [CrossRef]
- Shen, J.; Dudik, L.; Liu, C.-C. An iridium nanoparticles dispersed carbon based thick film electrochemical biosensor and its application for a single use, disposable glucose biosensor. Sens. Actuat. B Chem. 2007, 125, 106–113. [Google Scholar] [CrossRef]
- Rivas, G.A.; Rubianes, M.D.; Rodríguez, M.C.; Ferreyra, N.F.; Luque, G.L.; Pedano, M.L.; Miscoria, S.A.; Parrado, C. Carbon nanotubes for electrochemical biosensing. Talanta 2007, 74, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Sharpe, E.; Andreescu, S. Nanoparticle-Based Technologies for the Detection of Food Antioxidants. Curr. Anal. Chem. 2012, 8, 495–505. [Google Scholar] [CrossRef]
- Metters, J.P.; Houssein, S.M.; Kampouris, D.K.; Banks, C.E. Paper-based electroanalytical sensing platforms. Anal. Methods 2013, 5, 103–110. [Google Scholar] [CrossRef]
- Abe, K.; Kotera, K.; Suzuki, K.; Citterio, D. Inkjet-printed paperfluidic immuno-chemical sensing device. Anal. Bioanal. Chem. 2010, 398, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Mazumdar, D.; Lu, Y. A simple and sensitive “dipstick” test in serum based on lateral flow separation of aptamer-linked nanostructures. Angew. Chem. Int. Ed. 2006, 45, 7955–7959. [Google Scholar] [CrossRef] [PubMed]
- Alkasir, R.S.; Rossner, A.; Andreescu, S. Portable Colorimetric Paper-Based Biosensing Device for the Assessment of Bisphenol A in Indoor Dust. Environ. Sci. Technol. 2015, 49, 9889–9897. [Google Scholar] [CrossRef] [PubMed]
- Alkasir, R.S.; Ganesana, M.; Won, Y.H.; Stanciu, L.; Andreescu, S. Enzyme functionalized nanoparticles for electrochemical biosensors: A comparative study with applications for the detection of bisphenol, A. Biosens. Bioelectron. 2010, 26, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal. Chim. Acta 2010, 674, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical Detection for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Desmet, C.; Marquette, C.A.; Blum, L.J.; Doumèche, B. Paper electrodes for bioelectrochemistry: Biosensors and biofuel cells. Biosens. Bioelectron. 2016, 76, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Liana, D.D.; Raguse, B.; Wieczorek, L.; Baxter, G.R.; Chuah, K.; Gooding, J.J.; Chow, E. Sintered gold nanoparticles as an electrode material for paper-based electrochemical sensors. RSC Adv. 2013, 3, 8683–8691. [Google Scholar] [CrossRef]
- Nie, Z.; Deiss, F.; Liu, X.; Akbulut, O.; Whitesides, G.M. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip 2010, 10, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Hua, F.; Schuckers, S.; Andreescu, S.; Bradley, R. Effects of brewing conditions on the antioxidant capacity of twenty-four commercial green tea varieties. Food Chem. 2016, 192, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yaktine, A.; Pray, L. Nanotechnology in Food Products: Workshop Summary; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Xihong, Z.; Chii-Wann, L.; Jun, W.; Deog Hwan, O. Advances in rapid detection methods for foodborne pathogens. J. Microbiol. Biotechnol. 2014, 24, 297–312. [Google Scholar]
- Poltronieri, P.; Mezzolla, V.; Primiceri, E.; Maruccio, G. Biosensors for the Detection of Food Pathogens. Foods 2014, 3, 511–526. [Google Scholar] [CrossRef]
- Jokerst, J.C.; Adkins, J.A.; Bisha, B.; Mentele, M.M.; Goodridge, L.D.; Henry, C.S. Development of a paper-based analytical device for colorimetric detection of select. Foodborne Pathog. Anal. Chem. 2012, 84, 2900–2907. [Google Scholar]
- Jodi Woan-Fei, L.; Ab Mutalib, N.-S.; Chan, K.-G.; Lee, L.-H. Rapid Methods for the Detection of Foodborne Bacterial Pathogens: Principles, Applications, Advantages and Limitations. Front. Microbiol. 2015, 5. [Google Scholar] [CrossRef]
- Hossain, S.Z.; Ozimok, C.; Sicard, C.; Aguirre, S.D.; Ali, M.M.; Li, Y.; Brennan, J.D. Multiplexed paper test strip for quantitative bacterial detection. Anal. Bioanal. Chem. 2012, 403, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Sodha, S.; Heiman, K.; Gould, L.; Bishop, R.; Iwamoto, M.; Swerdlow, D.; Griffin, P.M. National patterns of Escherichia coli O157 infections, USA, 1996–2011. Epidemiol. Infect. 2015, 143, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Shipman, L.; Greene, K.; Sowers, E.; Green, J.; Cameron, D.; Downes, F.P.; Martin, M.L.; Griffin, P.M.; Ostroff, S.M.; et al. Isolation of Escherichia coli serotype O157: H7 and other Shiga-like-toxin-producing, E. coli from dairy cattle. J. Clin. Microbiol. 1991, 29, 985–959. [Google Scholar] [PubMed]
- Feng, P. Escherichia coli serotype O157: H7: Novel vehicles of infection and emergence of phenotypic variants. Emerg. Infect. Dis. 1995, 1, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y. An Antibody-Immobilized Capillary Column as a Bioseparator/Bioreactor for Detection of Escherichia coli O157:H7 with Absorbance Measurement. Anal. Chem. 2001, 73, 5180–5183. [Google Scholar] [CrossRef] [PubMed]
- Ngom, B.; Guo, Y.; Wang, X.; Bi, D. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: A review. Anal. Bioanal. Chem. 2010, 397, 1113–1135. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.Y.; Jung, S.C.; Kweon, C.H. Development of a rapid immunochromatographic strip for detection of Escherichia coli O157. J. Food Prot. 2005, 68, 2140–2143. [Google Scholar] [PubMed]
- Wutor, V.; Togo, C.; Pletschke, B. Suitability of total coliform β-d-galactosidase activity and CFU counts in monitoring faecal contamination of environmental water samples. Water SA 2009, 35, 85–88. [Google Scholar]
- Kilian, M.; Bülo, P. Rapid Diagnosis Of Enterobacteriaceae. Acta Pathol. Microbiol. Scand. Sec. B Microbiol. 1976, 84, 245–251. [Google Scholar] [CrossRef]
- Ratnam, S.; March, S.B.; Ahmed, R.; Bezanson, G.; Kasatiya, S. Characterization of Escherichia coli serotype O157:H7. J. Clin. Microbiol. 1988, 26, 2006–2012. [Google Scholar] [PubMed]
- Stephen Inbaraj, B.; Chen, B.H. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food Drug Anal. 2015, in press. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Lo Fo Wong, D.M.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, G.; Brandi, G.; Schiavano, G.F. Incidence and role of Salmonella in seafood safety. Food Res. Int. 2012, 45, 780–788. [Google Scholar] [CrossRef]
- Joo, J.; Yim, C.; Kwon, D.; Lee, J.; Shin, H.H.; Cha, H.J.; Jeon, S. A facile and sensitive detection of pathogenic bacteria using magnetic nanoparticles and optical nanocrystal probes. Analyst 2012, 137, 3609–3612. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Wang, Y.-F.; Yan, X.-P. Amine-Functionalized Magnetic Nanoparticles for Rapid Capture and Removal of Bacterial Pathogens. Environ. Sci. Technol. 2010, 44, 7908–7913. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Fareedullah, M.; Sudhakar, Y.; Venkateswarlu, B.; Kumar, E.A. Current review on organophosphorus poisoning. Arch. Appl. Sci. Res. 2010, 2, 199–215. [Google Scholar]
- Andreescu, S.; Marty, J.L. Twenty years research in cholinesterase biosensors: From basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.N. Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J. Toxicol. Environ. Health B Crit. Rev. 1999, 2, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Valdés, M.; Valdés González, A.; García Calzón, J.; Díaz-García, M. Analytical nanotechnology for food analysis. Microchim. Acta 2009, 166, 1–19. [Google Scholar] [CrossRef]
- Pérez-López, B.; Merkoçi, A. Nanomaterials based biosensors for food analysis applications. Trends Food Sci. Technol. 2011, 22, 625–639. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Hossain, S.M.Z.; Luckham, R.E.; Smith, A.M.; Lebert, J.M.; Davies, L.M.; Pelton, R.H.; Filipe, C.D.; Brennan, J.D. Development of a Bioactive Paper Sensor for Detection of Neurotoxins Using Piezoelectric Inkjet Printing of Sol-Gel-Derived Bioinks. Anal. Chem. 2009, 81, 5474–5483. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.Z.; Luckham, R.E.; McFadden, M.J.; Brennan, J.D. Reagentless Bidirectional Lateral Flow Bioactive Paper Sensors for Detection of Pesticides in Beverage and Food Samples. Anal. Chem. 2009, 81, 9055–9064. [Google Scholar] [CrossRef] [PubMed]
- Luckham, R.E.; Brennan, J.D. Bioactive paper dipstick sensors for acetylcholinesterase inhibitors based on sol-gel/enzyme/gold nanoparticle composites. Analyst 2010, 135, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Fan, K.; Pan, Y.; Jiang, H.; Wang, F.; Yang, D.; Lu, D.; Feng, J.; Zhao, J.; Yang, L.; et al. Fe3O4 Magnetic Nanoparticle Peroxidase Mimetic-Based Colorimetric Assay for the Rapid Detection of Organophosphorus Pesticide and Nerve Agent. Anal. Chem. 2013, 85, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Barry, R.; Petersen, C.; Timchalk, C.; Gassman, P.L.; Lin, Y. Nanoparticle-based electrochemical immunosensor for the detection of phosphorylated acetylcholinesterase: An exposure biomarker of organophosphate pesticides and nerve agents. Chemistry 2008, 14, 9951–9959. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, B.; Li, W.; Yu, H. Silver nanoparticle-based chemiluminescent sensor array for pesticide discrimination. J. Agric. Food Chem. 2015, 63, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhong, G.; Kim, D.-E.; Liu, J.; Liu, X. A portable lab-on-a-chip system for gold-nanoparticle-based colorimetric detection of metal ions in water. Biomicrofluidics 2014, 8, 052107. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Z.; Fan, J.; Peng, X. Gold nanoparticle-based colorimetric detection of mercury ion via coordination chemistry. Sens. Actuat. B Chem. 2015, 212, 481–486. [Google Scholar] [CrossRef]
- Chansuvarn, W.; Tuntulani, T.; Imyim, A. Colorimetric detection of mercury(II) based on gold nanoparticles, fluorescent gold nanoclusters and other gold-based nanomaterials. TrAC Trends Anal. Chem. 2015, 65, 83–96. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, H.; Liu, L.; Li, M.; Xiao, K.; Xu, M. Selective and sensitive colorimetric sensor of mercury (II) based on gold nanoparticles and 4-mercaptophenylboronic acid. Sens. Actuat. B Chem. 2014, 196, 106–111. [Google Scholar] [CrossRef]
- Chen, G.-H.; Chen, W.-Y.; Yen, Y.-C.; Wang, C.-W.; Chang, H.-T.; Chen, C.-F. Detection of Mercury(II) Ions Using Colorimetric Gold Nanoparticles on Paper-Based Analytical Devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Uzun, L.; Denizli, A. Colorimetric Sensor Array Based on Gold Nanoparticles and Amino Acids for Identification of Toxic Metal Ions in Water. ACS Appl. Mater. Interfaces 2014, 6, 18395–18400. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Khodaei, M.M.; Hamidi, Z.; Shamsuddin, M.B. Naked-eye colorimetric detection of Cu2+ and Ag+ ions based on close-packed aggregation of pyridines-functionalized gold nanoparticles. Sens. Actuat. B Chem. 2014, 190, 782–791. [Google Scholar] [CrossRef]
- Annadhasan, M.; Muthukumarasamyvel, T.; Sankar Babu, V.R.; Rajendiran, N. Green Synthesized Silver and Gold Nanoparticles for Colorimetric Detection of Hg2+, Pb2+, and Mn2+ in Aqueous Medium. ACS Sustain. Chem. Eng. 2014, 2, 887–896. [Google Scholar] [CrossRef]
- Sung, Y.-M.; Wu, S.-P. Colorimetric detection of Cd(II) ions based on di-(1H-pyrrol-2-yl)methanethione functionalized gold nanoparticles. Sens. Actuat. B Chem. 2014, 201, 86–91. [Google Scholar] [CrossRef]
- Cheli, F.; Pinotti, L.; Campagnoli, A.; Fusi, E.; Rebucci, R.; Baldi, A. Mycotoxin Analysis, Mycotoxin-Producing Fungi Assays and Mycotoxin Toxicity Bioassays in Food Mycotoxin Monitoring and Surveillance. Ital. J. Food Sci. 2008, 20, 447–462. [Google Scholar]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Fernández-Cruz, M.L.; Mansilla, M.L.; Tadeo, J.L. Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. J. Adv. Res. 2010, 1, 113–122. [Google Scholar] [CrossRef]
- Hosseini, M.; Khabbaz, H.; Dadmehr, M.; Ganjali, M.R.; Mohamadnejad, J. Aptamer-Based Colorimetric and Chemiluminescence Detection of Aflatoxin B1 in Foods Samples. Acta Chim. Slov. 2015, 62, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Chen, J.; Xie, G.; Li, C.; Ping, H.; Ma, Z.; Lu, A. Visual and microplate detection of aflatoxin B2 based on NaCl-induced aggregation of aptamer-modified gold nanoparticles. Microchim. Acta 2015, 182, 995–1001. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, D.; Lin, Z.; Qiu, B.; Liu, M.; Guo, L.; Chen, G. Disassembly of gold nanoparticle dimers for colorimetric detection of ochratoxin, A. Anal. Methods 2015, 7, 842–825. [Google Scholar] [CrossRef]
- Soh, J.H.; Lin, Y.; Rana, S.; Ying, J.Y.; Stevens, M.M. Colorimetric Detection of Small Molecules in Complex Matrixes via Target-Mediated Growth of Aptamer-Functionalized Gold Nanoparticles. Anal. Chem. 2015, 87, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niessner, R.; Knopp, D. Magnetic Bead-Based Colorimetric Immunoassay for Aflatoxin B1 Using Gold Nanoparticles. Sensors 2014, 14, 21535–21548. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.E.; Petrakova, A.V.; Vozniak, M.V.; Zherdev, A.V.; Dzantiev, B.B. Rapid Immunoenzyme Assay of Aflatoxin B1 Using Magnetic Nanoparticles. Sensors 2014, 14, 21843–21857. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Volpe, G.; Micheli, L.; Palleschi, G. A review on novel developments and applications of immunosensors in food analysis. Anal. Chim. Acta 2007, 605, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Mishra, R.; Catanante, G.; Marty, J. Development of an aptasensor based on a fluorescent particles-modified aptamer for ochratoxin A detection. Anal. Bioanal. Chem. 2015, 407, 7815–7822. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bülbül, G.; Hayat, A.; Andreescu, S. Portable Nanoparticle-Based Sensors for Food Safety Assessment. Sensors 2015, 15, 30736-30758. https://doi.org/10.3390/s151229826
Bülbül G, Hayat A, Andreescu S. Portable Nanoparticle-Based Sensors for Food Safety Assessment. Sensors. 2015; 15(12):30736-30758. https://doi.org/10.3390/s151229826
Chicago/Turabian StyleBülbül, Gonca, Akhtar Hayat, and Silvana Andreescu. 2015. "Portable Nanoparticle-Based Sensors for Food Safety Assessment" Sensors 15, no. 12: 30736-30758. https://doi.org/10.3390/s151229826






