High Resolution Viscosity Measurement by Thermal Noise Detection
Abstract
:1. Introduction
2. Theory
| (mPa·s) | kg/m | |
|---|---|---|
| 0 | 0.9135 | 997.1 |
| 10 | 1.2501 | 1029.7 |
| 20 | 1.7784 | 1060.6 |
| 30 | 2.6529 | 1090.0 |
| 40 | 4.1971 | 1118.0 |
| 50 | 7.1505 | 1144.7 |
3. Setup
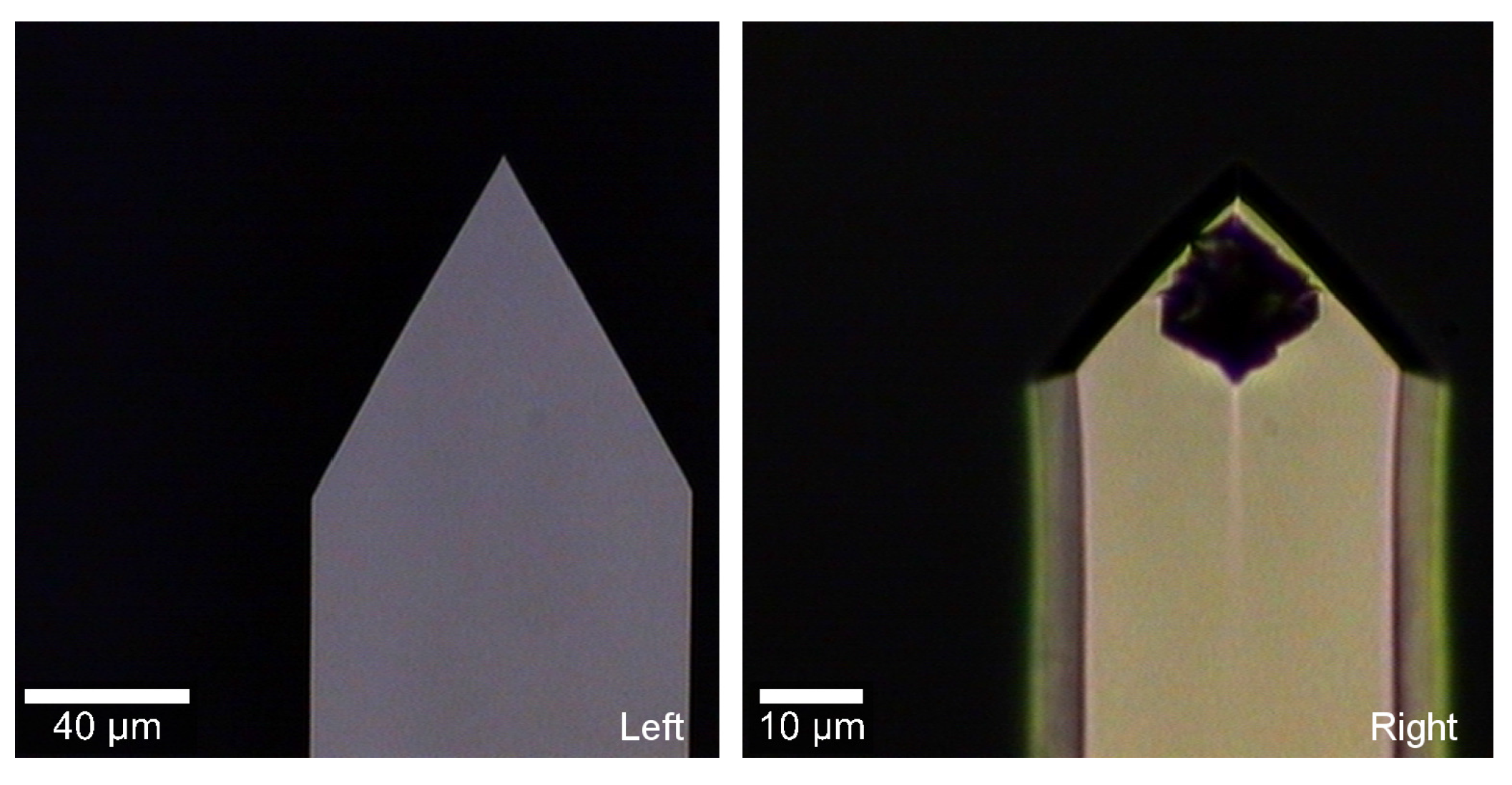
3.1. Sensing
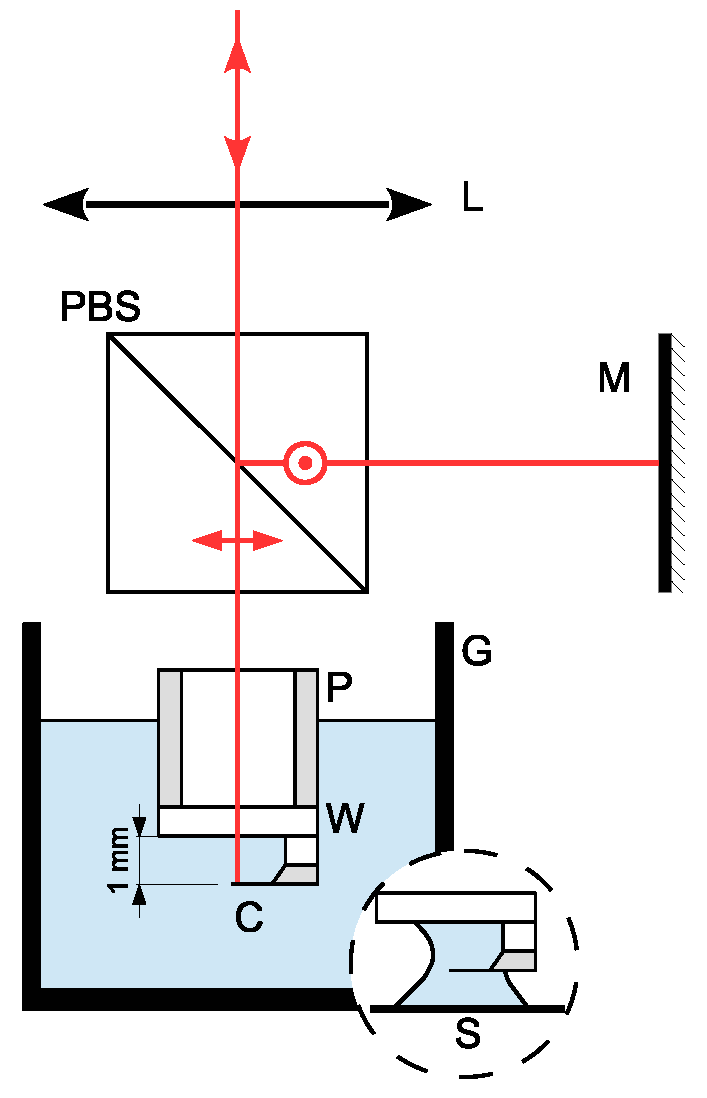
3.2. Immersion Area and Fluid Cell
4. Methods
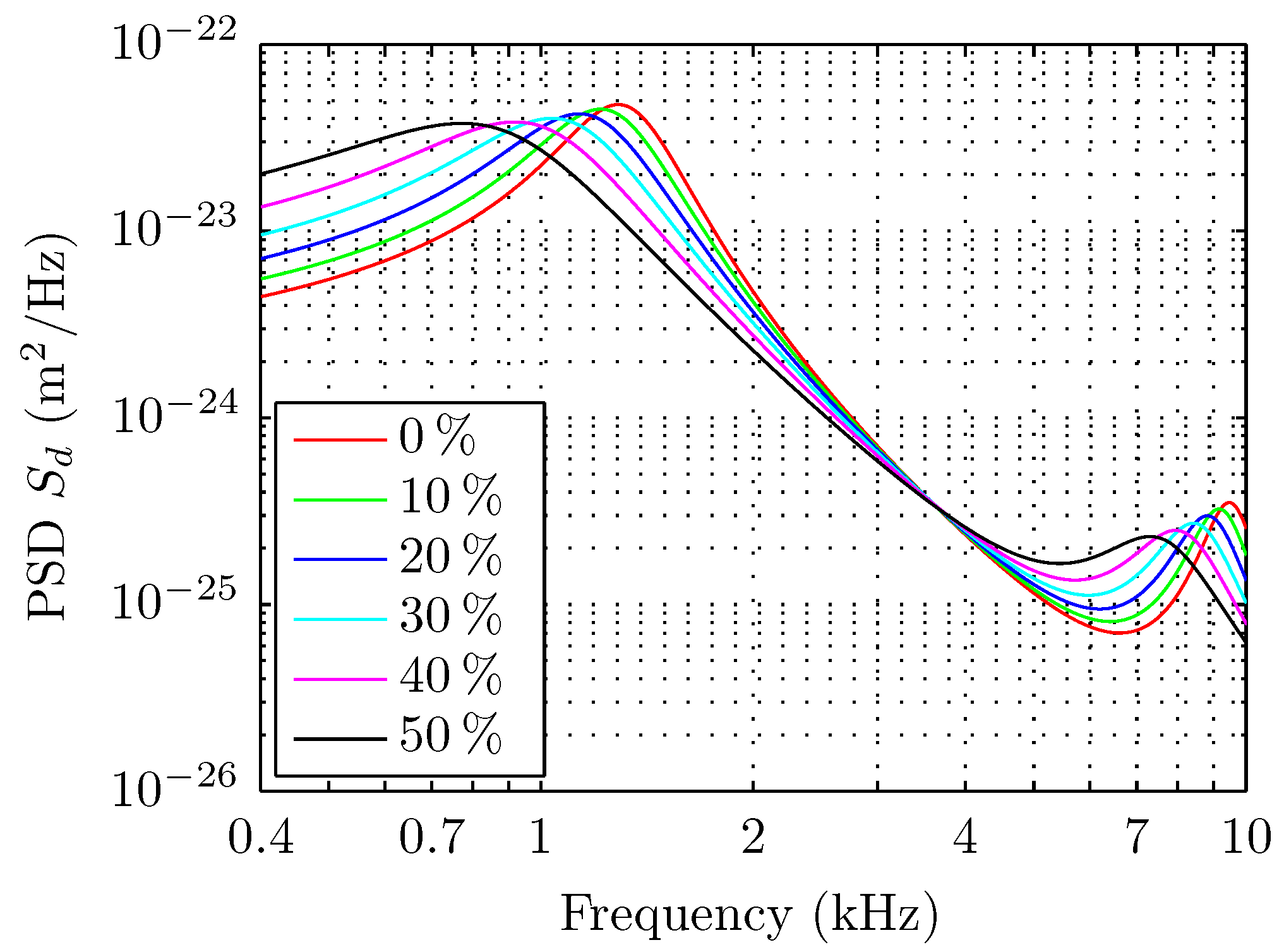
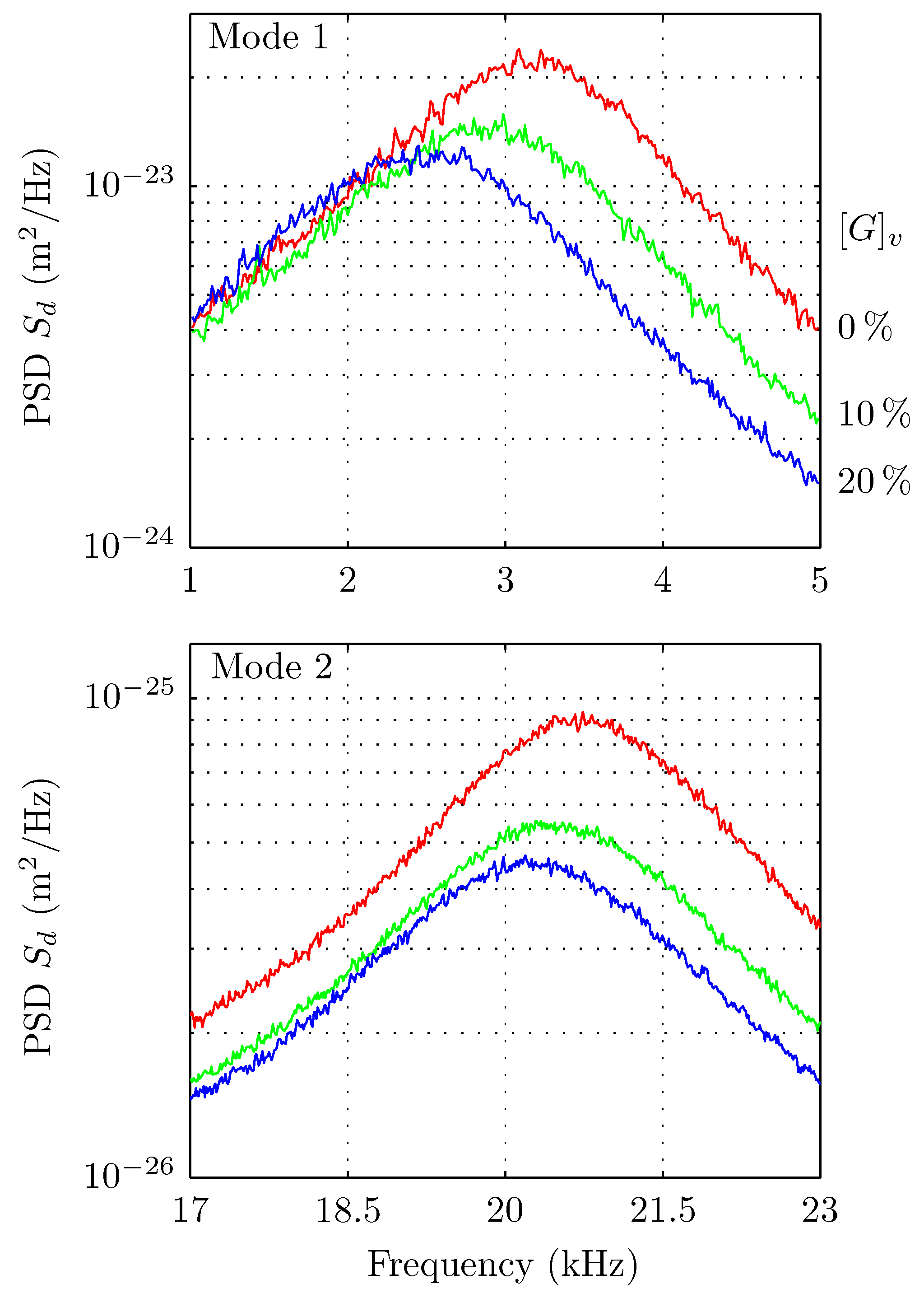
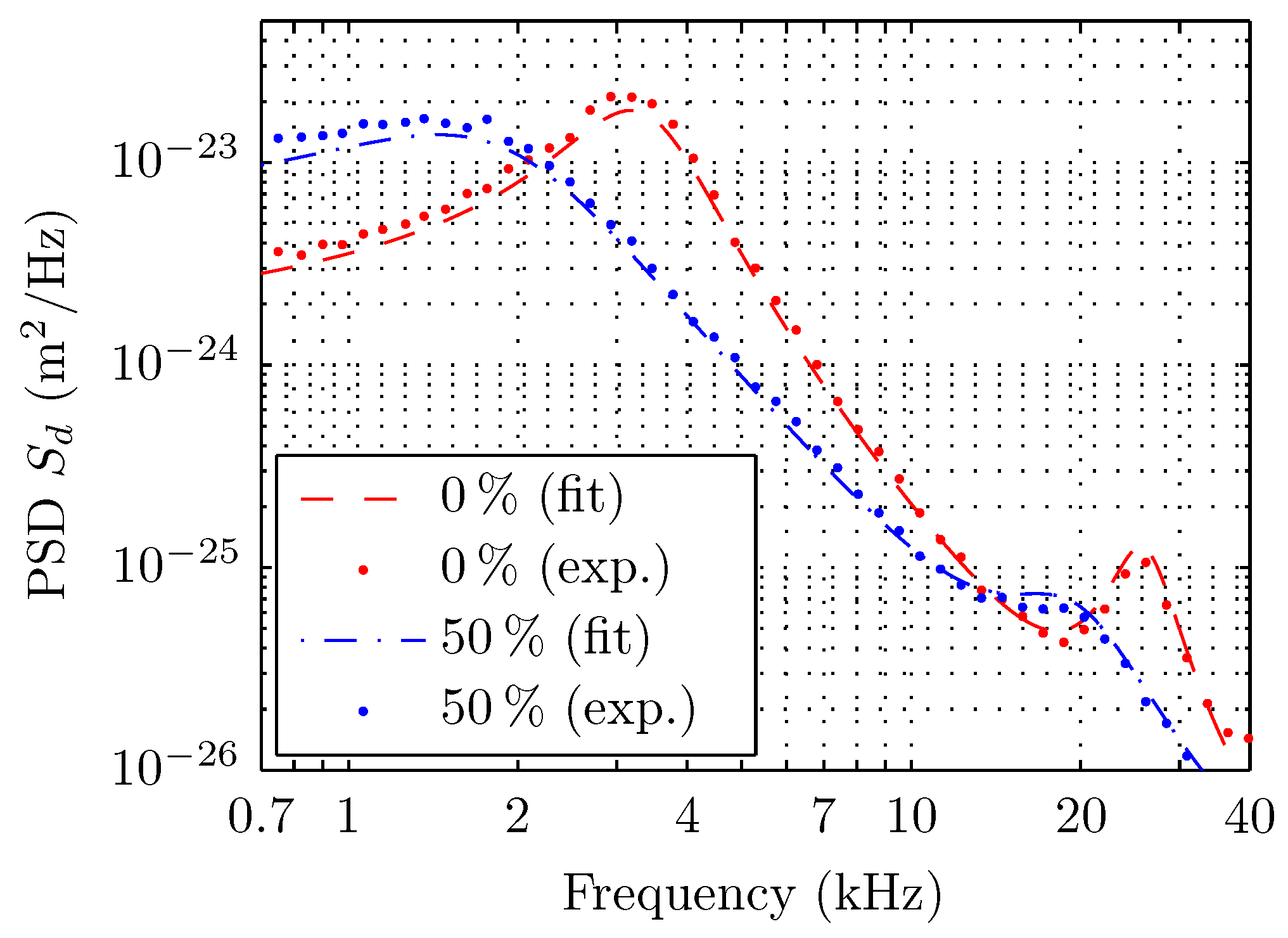
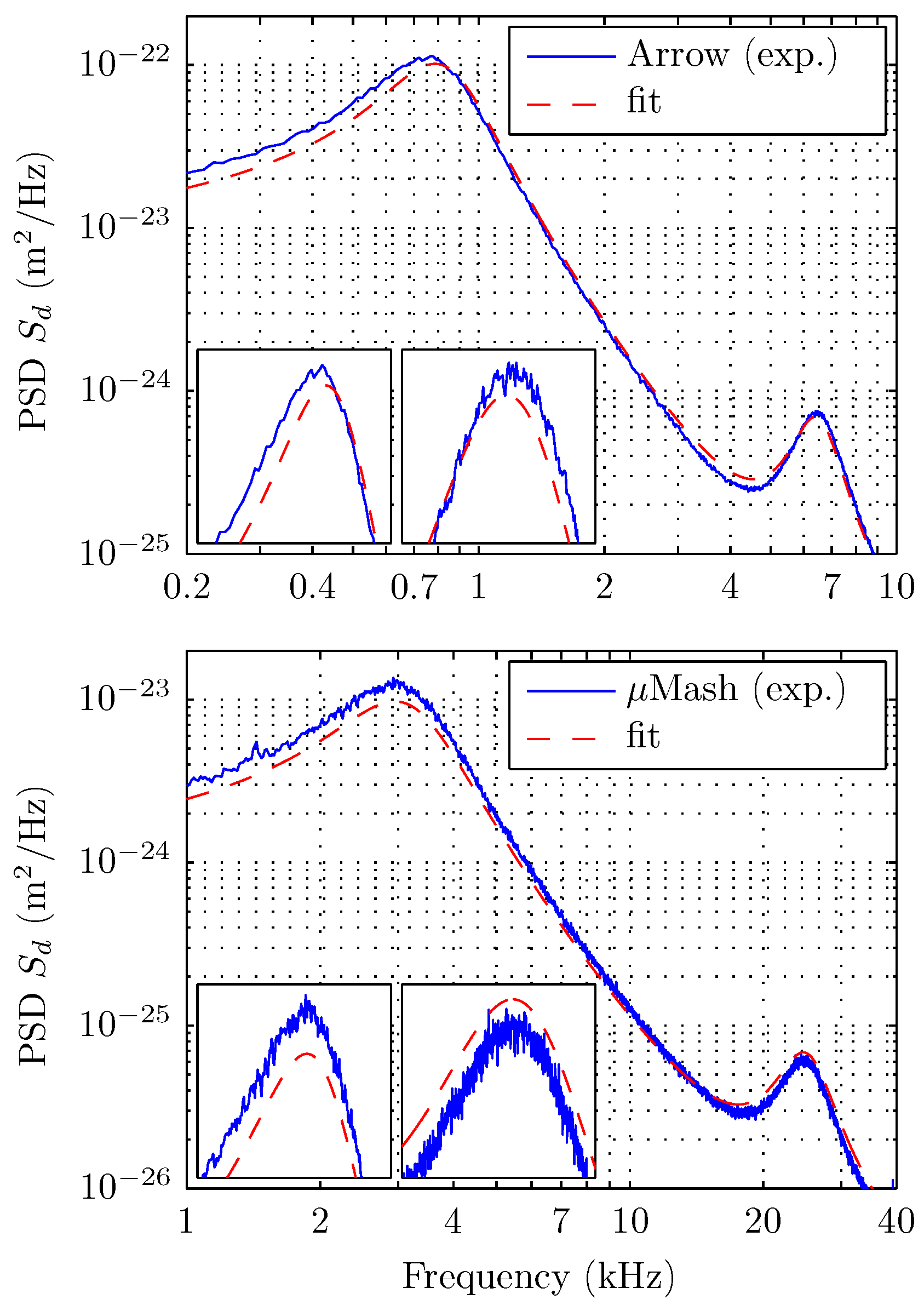
5. Results
| (mPa·s) | (mPa·s) | error (%) | |
|---|---|---|---|
| 0 | 0.913 | 0.912 | −0.07 |
| 1 | 0.938 | 0.940 | 0.16 |
| 2 | 0.967 | 0.957 | −1.11 |
| 3 | 0.995 | 0.993 | −0.23 |
| 4 | 1.026 | 1.029 | 0.30 |
| 5 | 1.058 | 1.062 | 0.38 |
| 6 | 1.092 | 1.096 | 0.35 |
| 7 | 1.125 | 1.125 | 0.04 |
| 8 | 1.162 | 1.177 | 1.34 |
| 9 | 1.200 | 1.212 | 0.98 |
| (mPa·s) | (mPa·s) | error (%) | |
|---|---|---|---|
| 10 | 1.256 | 1.278 | 1.7 |
| 20 | 1.783 | 1.785 | 0.1 |
| 30 | 2.661 | 2.580 | –3.0 |
| 40 | 4.197 | 4.047 | –3.6 |
| 50 | 7.150 | 6.573 | –8.1 |
6. Discussions
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schilowitz, A. Microcantilever Sensors for Petrochemical Applications. In Scanning Probe Microscopy for Industrial Applications: Nanomechanical Characterization; Yablon, D., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 251–269. [Google Scholar]
- Roberts, I. In-line and on-line rheology measurement. In Instrumentation and Sensors for the Food Industry; Kress-Rogers, E., Brimelow, C., Eds.; CRC Press: Cambridge, UK, 2001; pp. 403–422. [Google Scholar]
- Harrison, C.; Ryu, S.; Goodwin, A.; Hsu, K.; Donzier, E.; Marty, F.; Mercier, B. A MEMS sensor for the measurement of density-viscosity for oilfield applications. SPIE Proc. 2006, 6111, 1–11. [Google Scholar]
- Cao-Paz, A.; Rodríguez-Pardo, L.; Fariña, J.; Marcos-Acevedo, J. Resolution in QCM Sensors for the Viscosity and Density of Liquids: Application to Lead Acid Batteries. Sensors 2012, 12, 10604–10620. [Google Scholar] [CrossRef] [PubMed]
- Boss, C.; Meurville, E.; Sallese, J.; Ryser, P. A viscosity-dependent affinity sensor for continuous monitoring of glucose in biological fluids. Biosens. Bioelectron. 2011, 30, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, O.; Elbuken, C.; Ermek, E.; Mostafazadeh, A.; Baris, I.; Erdem Alaca, B.; Halil Kavakli, I.; Urey, H. Microcantilever based disposable viscosity sensor for serum and blood plasma measurements. Methods 2013, 63, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C. Measurement and interpretation of synovial fluid viscosities. Ann. Rheum. Dis. 1958, 17, 234–239. [Google Scholar] [CrossRef]
- Jalili, N.; Laxminarayana, K. A review of atomic force microscopy imaging systems: Application to molecular metrology and biological sciences. Mechatronics 2004, 14, 907–945. [Google Scholar] [CrossRef]
- Dufrene, Y.; Martinez-Martin, D.; Medalsy, I.; Alsteens, D.; Muller, D. Multiparametric imaging of biological systems by force-distance curve-based AFM. Nat. Methods 2013, 10, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.; Capella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- Hodges, C. Measuring forces with the AFM: Polymeric surfaces in liquids. Adv. Colloid Interface Sci. 2002, 99, 13–75. [Google Scholar] [CrossRef]
- Neuman, R.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Kadaa, G.; Kienbergera, F.; Hinterdorferb, P. Atomic force microscopy in bionanotechnology. Nanotoday 2008, 3, 12–19. [Google Scholar] [CrossRef]
- Lavrik, N.; Sepaniak, M.; Panos, G.D. Cantilever transducers as a platform for chemical and biological sensors. Rev. Sci. Instrum. 2004, 75, 2229–2253. [Google Scholar] [CrossRef]
- Tamayo, J.; Kosaka, P.M.; Ruz, J.J.; San Paulo, A.; Calleja, M. Biosensors based on nanomechanical systems. Chem. Soc. Rev. 2013, 42, 1287–1311. [Google Scholar] [CrossRef] [PubMed]
- Hutter, J.L.; Bechhoeffer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Butt, H.; Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 1995, 6, 1–7. [Google Scholar] [CrossRef]
- Stark, R.; Drobek, T.; Heckl, W. Thermomechanical noise of a free v-shaped cantilever for atomic-force microscopy. Ultramicroscopy 2001, 86, 207–215. [Google Scholar] [CrossRef]
- Paolino, P.; Aguilar Sandoval, F.; Bellon, L. Quadrature phase interferometer for high resolution force spectroscopy. Rev. Sci. Instrum. 2013, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, R.; Aguilar Sandoval, F.; Wilson, C.A.M.; Melo, F. Pulling on super paramagnetic beads with micro cantilevers: single molecule mechanical assay application. Phys. Biol. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Sader, J.E. Frequency response of cantilever beams immersed in viscous fluids with applications to the atomic force microscope. J. Appl. Phys. 1998, 84, 64–76. [Google Scholar] [CrossRef]
- Bergaud, C.; Nicu, L. Viscosity measurements based on experimental investigations of composite cantilever beam eigenfrequencies in viscous media. Rev. Sci. Instrum. 2000, 71, 2487–2491. [Google Scholar] [CrossRef]
- Papi, M.; Arcovito, G.; de Spirito, M.; Vassalli, M.; Tiribilli, B. Fluid viscosity determination by means of uncalibrated atomic force microscopy cantilevers. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- McLoughlin, N.; Lee, S.L.; Hahner, G. Temperature dependence of viscosity and density of viscous liquids determined from thermal noise spectra of uncalibrated atomic force microscope cantilevers. Lab Chip 2007, 7, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Hennemeyer, M.; Burghardt, S.; Stark, R.W. Cantilever Micro-rheometer for the Characterization of Sugar Solutions. Sensors 2008, 8, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Paxman, R.; Stinson, J.; Dejardin, A.; McKendry, R.A.; Hoogenboom, B.W. Using Micromechanical Resonators to Measure Rheological Properties and Alcohol Content of Model Solutions and Commercial Beverages. Sensors 2012, 12, 6497–6507. [Google Scholar] [CrossRef] [PubMed]
- Bonfig, K. Das Direkte Digitale Messverfahren als Grundlage einfacher und dennoch genauer und storsicherer Sensoren. Sensor 1988, 3, 103–108. [Google Scholar]
- Matko, V.; Safaric, R. Major Improvements of Quartz Crystal Pulling Sensitivity and Linearity Using Series Reactance. Sensors 2009, 9, 8263–8270. [Google Scholar] [CrossRef] [PubMed]
- Matko, V. Next Generation AT-Cut Quartz Crystal Sensing Devices. Sensors 2011, 11, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- Bellon, L. Thermal noise of microcantilevers in viscous fluids. J. Appl. Phys. 2008, 104. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N. Formula for the Viscosity of a Glycerol Water Mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar Sandoval, F.; Sepúlveda, M.; Bellon, L.; Melo, F. High Resolution Viscosity Measurement by Thermal Noise Detection. Sensors 2015, 15, 27905-27916. https://doi.org/10.3390/s151127905
Aguilar Sandoval F, Sepúlveda M, Bellon L, Melo F. High Resolution Viscosity Measurement by Thermal Noise Detection. Sensors. 2015; 15(11):27905-27916. https://doi.org/10.3390/s151127905
Chicago/Turabian StyleAguilar Sandoval, Felipe, Manuel Sepúlveda, Ludovic Bellon, and Francisco Melo. 2015. "High Resolution Viscosity Measurement by Thermal Noise Detection" Sensors 15, no. 11: 27905-27916. https://doi.org/10.3390/s151127905





