Determination of the Mineral Composition and Toxic Element Contents of Propolis by Near Infrared Spectroscopy
Abstract
:1. Introduction
2. Experimental
2.1. Propolis Samples
2.2. Chemical Analysis of the Mineral Composition
2.3. NIR Spectroscopy
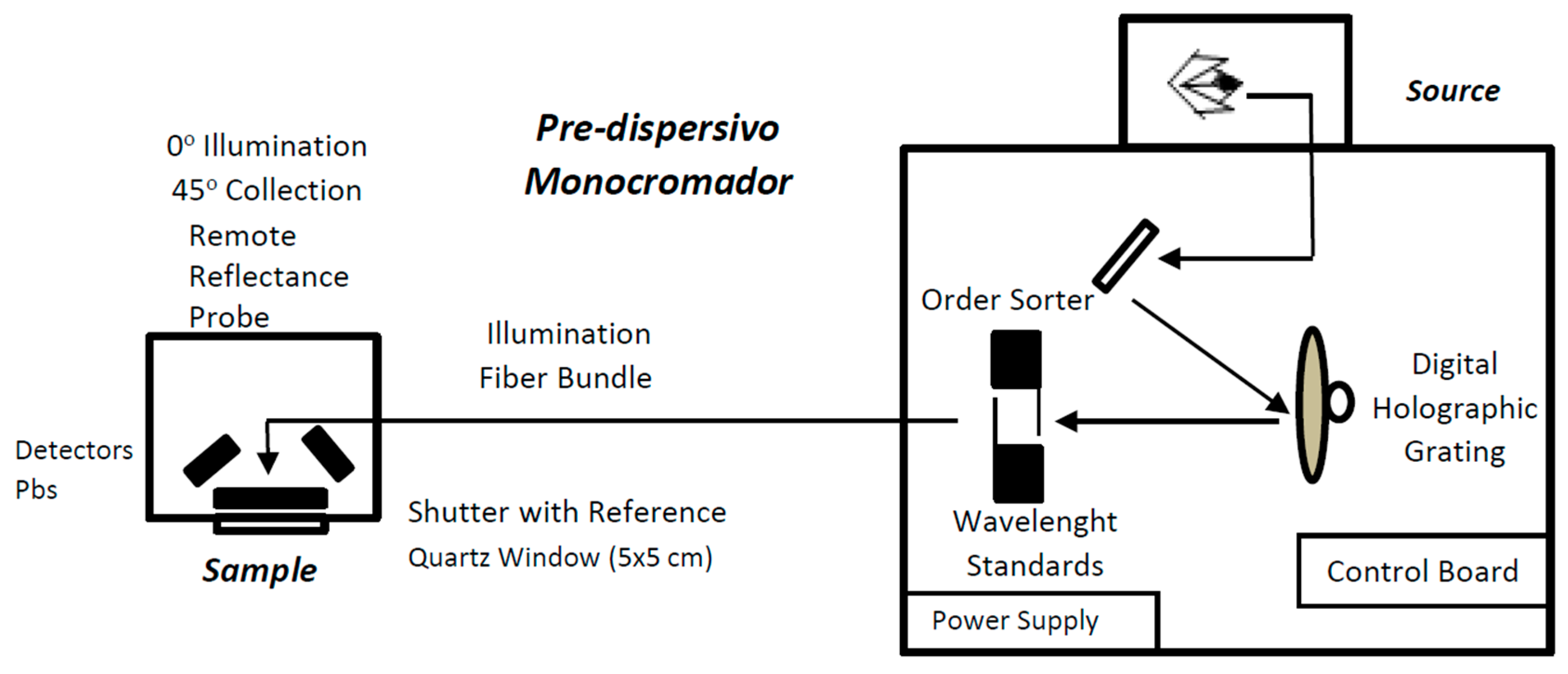
2.4. Statistical Data Analysis
2.4.1. Principal Component Analysis
2.4.2. Modified Partial Least Squares Regression
3. Results and Discussion
3.1. Chemical Composition
| Constituent | Total (N = 91) | Chile (N = 52) | Galicia (N = 16) | Castilla-León (N = 23) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| Al | 275.2 | 43.0–833.9 | 354.9a | 156.0–833.9 | 105.2b | 43.0–193.7 | 213.2c | 78.6–518.4 |
| Ca | 833.4 | 219.1–5173.0 | 910.4 | 274.0–5173.0 | 563.2 | 219.1–1176.1 | 847.3 | 416.7–2169.2 |
| Fe | 424.6 | 46.1–1538.0 | 536.6a | 181.8–1538.0 | 245.8b | 46.1–656.7 | 295.8b | 104.5–874.0 |
| K | 978.6 | 267.0–4428.3 | 550.0a | 267.0–1841.2 | 1522.1b | 359.0–3182.1 | 1569.4b | 685.9–4428.3 |
| Mg | 234.1 | 63.5–1398.0 | 261.8 | 75.1–1398.0 | 206.4 | 63.5–427.0 | 190.6 | 88.2–460.3 |
| P | 235.0 | 116.0–729.0 | 228.8a | 118.1–402.0 | 307.5b | 152.3–729.0 | 198.5a | 116.0–327.7 |
| Cr | 3.7 | 0.8–48.9 | 3.1 | 1.4–5.5 | 2.7 | 0.8–7.5 | 5.7 | 2.3–48.9 |
| Cu | 1.8 | Nd–33.4 | 1.6a | Nd–6.2 | 5.4b | Nd–33.4 | 2.8 | Nd–7.2 |
| Ni | 1.5 | Nd–29.9 | 1.2 | Nd–9.7 | 1.4 | 0.5–4.0 | 2.4 | 0.6–29.9 |
| Pb | 5.8 | Nd–73.9 | 2.6a | Nd–8.0 | 2.2a | Nd–6.0 | 15.5b | Nd–74.0 |
| Zn | 62.6 | 5.5–460.7 | 57.8 | 5.5–105.0 | 89.8 | 17.4–460.7 | 54.4 | 11.1–145.3 |
| Constituent | South Spain [25] (N = 25) | Argentina [5] (N = 10) | Argentina [10] (N = 96) | China [11] (N = 32) | Brazil [26] (N = 42) |
|---|---|---|---|---|---|
| Al | 308–582 | – | – | 426–1959 | Nd–1840 |
| Ca | 1773–6683 | 39–4138 | – | 404–2637 | Nd–4800 |
| Fe | 312–1270 | 101–1697 | 400–1945 | 310–2125 | – |
| K | 735–4790 | 101–1697 | – | 314v1894 | 410–5490 |
| Mg | 301–1405 | 1115–1031 | – | 135–1129 | 500–4650 |
| P | 171–611 | – | – | – | – |
| Cr | 0.3–3 | Nd | 0.6–3.7 | Nd–12 | Nd–19 |
| Cu | 2.1–4 | Nd | – | Nd–15 | Nd |
| Ni | 0.6–3 | Nd | – | Nd–3 | – |
| Pb | 0.07–4 | – | – | 4–55 | Nd–160 |
| Zn | 163–1236 | 33-147 | 11–105 | 35–386 | Nd–500 |
| Constituent | Poland [37,38] (N = 20) | Croatia [39] | Poland [12] (N = 80) | Turkey [40] | |
| Al | – | – | – | – | |
| Ca | – | 40–317 | – | 79–118 | |
| Fe | 28–101 | 14–251 | 101 | – | |
| K | – | 51–117 | 8.2 | 121–364 | |
| Mg | 137–823 | 10–46 | – | – | |
| P | – | – | – | – | |
| Cr | – | 0–1 | – | – | |
| Cu | – | 0.3–6 | – | 45–96 | |
| Ni | 2–10 | 0–0.3 | 9.8 | – | |
| Pb | 0.9–3 | 0.3–64 | 2.7 | – | |
| Zn | 18–71 | 8–933 | 71.5 | 176–676 |
3.2. NIR Calibration Equations
| Constituent | Math treatment | N | Mean | SD | Est. Min | Est. Max | RMSEC | R2 | RMECV | RPD |
|---|---|---|---|---|---|---|---|---|---|---|
| Al | Standard MSC 1,4,4,1 | 65 | 257.4 | 123.9 | 0.0 | 629.2 | 56.5 | 0.79 | 78.8 | 1.6 |
| Ca | None 1,4,4,1 | 60 | 509.4 | 245.9 | 0.0 | 1247.0 | 102.7 | 0.83 | 162.1 | 3.1 |
| Fe | None 1,4,4,1 | 64 | 425.6 | 231.0 | 0.0 | 1118.6 | 129.3 | 0.69 | 147.3 | 1.6 |
| K | Detrend only 2,4,4,1 | 58 | 772.8 | 559.6 | 0.0 | 2451.7 | 126.7 | 0.95 | 244.3 | 2.3 |
| Mg | None 1,4,4,1 | 64 | 198.4 | 193.7 | 0.0 | 779.4 | 105.6 | 0.70 | 160.6 | 1.2 |
| P | Standard MSC 1,4,4,1 | 66 | 236.7 | 103.9 | 0.0 | 548.4 | 26.0 | 0.94 | 40.4 | 2.6 |
| Cr | SNV only2,4,4,1 | 61 | 2.9 | 0.8 | 0.5 | 5.3 | 0.6 | 0.48 | 0.8 | 1.0 |
| Cu | Detrend only 2,10,10,1 | 58 | 1.2 | 1.6 | 0.0 | 5.8 | 0.9 | 0.64 | 1.2 | 1.3 |
| Ni | None 0,0,1,1 | 67 | 1.2 | 1.0 | 0.0 | 4.2 | 0.7 | 0.52 | 1.0 | 1.0 |
| Pb | None 1,4,4,1 | 59 | 3.5 | 3.7 | 0.0 | 14.6 | 2.0 | 0.70 | 3.3 | 1.1 |
| Zn | SNV only 2,4,4,1 | 64 | 57.4 | 28.9 | 0.0 | 144.2 | 10.6 | 0.87 | 18.7 | 1.6 |
3.3. Validation
3.3.1. Internal Validation (Prediction)


3.3.2. External Validation
| Constituent | Mean | SD | Est. Min | Est. Max | RMSEP | RMSEP(C) | RPD |
|---|---|---|---|---|---|---|---|
| Al | 239.5 | 91.8 | 24.6 | 370.2 | 114 | 113.4 | 0.8 |
| Ca | 946.3 | 290.6 | 70.8 | 1283.0 | 106.5 | 116.2 | 2.5 |
| Fe | 392.9 | 164.7 | 97,3 | 745.5 | 164.2 | 168.5 | 1.0 |
| K | 1052.1 | 572.3 | 453.4 | 2503.5 | 250.3 | 258.2 | 2.2 |
| Mg | 198.4 | 157.6 | 63.7 | 441.8 | 157.1 | 165.3 | 1.0 |
| P | 237.0 | 83.3 | 77.4 | 364.7 | 48.1 | 46.1 | 1.8 |
| Cr | 3.1 | 0.64 | 1.7 | 3.9 | 0.92 | 0.90 | 0.7 |
| Cu | 1.37 | 1.4 | 0.2 | 4.5 | 1.5 | 1.6 | 0.9 |
| Ni | 1.4 | 0.6 | 0.1 | 2.4 | 1.3 | 1.3 | 0.5 |
| Pb | 4.4 | 3.1 | 1.2 | 14.0 | 1.2 | 1.4 | 2.2 |
| Zn | 55.4 | 28.6 | 7.1 | 128.2 | 18.3 | 24.1 | 1.2 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gómez-Caravaca, A.; Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006, 41, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Bogdanov, A.; Sabatini, A.G. Chemical Composition of European propolis: expected and unexpected results. Z. Naturforsch 2002, 57, 530–533. [Google Scholar] [CrossRef]
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine 2005, 12, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.; Tapia, A.; Luna, L.; Fabani, M.P.; Schmeda-Hlrschmann, G.; Podio, N.S.; Wunderlin, D.A.; Feresin, G.E. Mainflavonoids, DPPH activity, and metal content allow determination of the geographical origin of propolis from the province of San Juan (Argentina). J. Agric. Food Chem. 2009, 57, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Laskar, R.A.; Roy, N.; Begum, N.A. Antioxidant activity of Indian propolis and its chemical constituents. Food Chem. 2010, 122, 233–237. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, A.; Dandlen, A.; Cavaco, A.M.; Antunes, M.D. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apismellifera L.) from Algarve, South Portugal. Food Sci. Technol. Camp. 2010, 34, 16–23. [Google Scholar]
- Sawaya, A.; Cunha, I.; Marcucci, M. Analytical methods applied to diverse types of Brazilian propolis. Chem. Cent. J. 2011, 5927, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Wu, S.W.; Ho, K.K.; Lin, S.B.; Huang, C.Y.; Chen, C.N. Characterisation of Taiwanese propolis collected from different locations and seasons. J. Sci. Food Agric. 2008, 88, 412–419. [Google Scholar] [CrossRef]
- Cantarelli, M.A.; Camiña, J.M.; Pettenati, E.M.; Marchevsky, E.J.; Pellerano, R.G. Trace mineral content of Argentinean raw propolis by neutron activation analysis (NAA): Assessment of geographical provenance by chemometrics. LWT-Food Sci. Technol. 2011, 44, 256–260. [Google Scholar] [CrossRef]
- Gong, S.; Luo, L.; Gong, W.; Gao, Y.; Sie, M. Multivariate analyses of element concentration revealed the grouping of propolis from different regions in China. Food Chem. 2012, 134, 583–588. [Google Scholar] [CrossRef]
- Formicki, G.; Gren, A.; Stawaez, R.; Zysk, B.; Gal, A. Metal content in honey, propolis, wax, and bee pollen and implication for metal pollution monitoring. Pol. J. Environ. Stud. 2013, 22, 99–106. [Google Scholar]
- Pierini, G.D.; Granero, A.M.; DiNezio, M.S.; Centurión, M.E.; Zon, M.A.; Femández, H. Development of an electroanalytical method for the determination of lead in Argentina raw propolis based on bismuth electrodes. Microchem. J. 2013, 106, 102–106. [Google Scholar] [CrossRef]
- Conti, M.E.; Botrè, F. Honeybees and their products as potential bioindicators of heavy metals contamination. Environ. Monit. Asses. 2001, 69, 267–282. [Google Scholar] [CrossRef]
- Alcici, N.; Freire, M. Heavy metals in propolis: Practical and simple procedures to reduce the lead level in the Brazilian propolis. In First German Bee Products and Apitherapy Congress; Plenum Press: New York, NY, USA, 2002. [Google Scholar]
- Falcó, G.; Llobet, J.M.; Zareba, S.; Krzysiak, K.; Domingo, J.L. Risk assessment of the trace elements intake through natural remedies in Poland. Trace Elem. Electrolytes 2005, 22, 222–226. [Google Scholar] [CrossRef]
- Ferreira, F.; Torres, A.; Rosa, O.; Ferreira, C.; Garcia, R.; Marcucci, M.; Gomes, B. Antimicrobial effect of propolis and other substances against selected endodontic pathogens. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Meers, E.; Ruttens, A.; Hopgood, M.J.; Samson, D.; Tack, F.M.G. Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextration of heavy metals. Chemosphere 2005, 58, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Visoottiviseth, P.; Francesconi, K.; Sridokchan, W. The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ. Pollut. 2002, 118, 453–461. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, Q.; Koval, P.V. Flowering stage characteristics of cadmium hyper accumulator Solanum nigrum L. and their significance to phyto remediation. Sci. Total Environ. 2006, 369, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Araújo, A.B.; Souza, S.C.; Santos E Silva, F.A. Study of the Cu, Mn, Pb and Zn dynamics in soil, plants and bee pollen from the region of Teresina (PI), Brazil. An. Acad. Bras. Ciênc. 2012, 84, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 167–202. [Google Scholar] [CrossRef]
- Sales, A.; Álvarez, A.; Rodriguez, M.; Maldonado, L.; Marchisio, P.; Rodríguez, M.; Bedascarrasbure, E. The effect of different propolis harvest methods on its lead contents determined by ET AAS and UV-vis. J. Hazard. Mater. 2006, 137, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Madras-Majewska, B.; Jasiński, Z. Lead content of bees, brood and bee products from different regions of Poland. J. Apic. Sci. 2003, 47, 47–55. [Google Scholar]
- Serra-Bonvehí, J.S.; Orantes-Bermejo, F.J.O. Element content of propolis collected from different areas of South Spain. Environ. Monit. Assess. 2013, 185, 6035–6047. [Google Scholar] [CrossRef] [PubMed]
- Finger, D.; Kelte-Filho, I.; Reyes-Torres, Y.; Pércio-Quináia, S. Propolis as an indicator of environmental contamination by metals. Bull. Environ. Contam. Toxicol. 2014, 92, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Xia, Z.; Sun, D.W.; He, Y. Application of visible and near infrared spectroscopy for rapid analysis of chrysin and galangin in Chinese propolis. Sensors 2013, 13, 10539–10549. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Cavaco, A.M.; Guerra, R.; Antunes, D.; Guia, H.; Miguel, M.G. A first approach to the optical and antioxidant properties of propolis collected at different sites of Algarve region. In Proceedings of the 4th IASME/WSEAS International Conference on Energy, Environment, Ecosystems and Sustainable Development (EEESD’08), Algarve, Portugal, 11 June 2008; pp. 112–114.
- Xu, L.; Yan, S.M.; Cai, C.B.; Yu, X.P. Untargeted detection and quantitative analysis of poplar balata (PB) in Chinese propolis by FT-NIR spectroscopy and chemometrics. Food Chem. 2013, 141, 4132–4137. [Google Scholar] [CrossRef] [PubMed]
- Hogendoorn, E.A.; Sommeijer, M.J.; Marjo, J.; Vredenbregt, M.J. Alternative method for measuring beeswax content in propolis from the Netherlands. J. Apic. Sci. 2013, 57, 81–90. [Google Scholar] [CrossRef]
- Muller, A.; Steinhart, H. Recent developments in instrumental analysis for food quality. Food Chem. 2006, 102, 436–444. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Sister, S.J.; Barnes, R.J. On the scales associated with near infrared reflectance difference spectra. Appl. Spectrosc. 1995, 49, 765–772. [Google Scholar] [CrossRef]
- Martens, H.; Naes, T. Multivariate Calibration; Wiley, Chichester: Oslo, Norway, 2001. [Google Scholar]
- Shenk, J.S.; Westhaus, M.O. Analysis of Agriculture and Food Products by Near Infrared Reflectance Spectroscopy. Monograph NIR Systems. Infrasoft International, NIR Systems. USA; Silver Spring Inc.: Silver Spring, MD, USA, 1995. [Google Scholar]
- Davies, A.M.C.; Williams, P. Near Infrared Spectroscopy: The Future Wawes. In Proceedings of the Seventh International Conference on Near Infrared Spectroscopy, West Sussex, UK, 12 September 1996; pp. 347–352.
- Williams, P.C.; Sobering, D. How do we do it: A brief summary of the methods we use in developing near infrared calibrations. In Near Infrared Spectroscopy: The Future Waves; Davies, A.M., Williams, C., Eds.; NIR Publications: Chichester, UK, 1996; pp. 185–188. [Google Scholar]
- Roman, A. Concentration of chosen trace elements of toxic properties in bee pollen loads. Pol. J. Environ. Stud. 2009, 18, 265–272. [Google Scholar]
- Roman, A. Level of copper, selenium, lead, and cadmium in Forager Bees. Pol. J. Environ. Stud. 2010, 19, 663–669. [Google Scholar]
- Cvek, J.; Medić-Šarić, M.; Vitali, D.; Vedrina-Dragojević, I.; Smit, Z.; Tomić, S. The content of essential and toxic elements in Croatian propolis samples and their tinctures. J. Apic. Res. 2008, 47, 35–45. [Google Scholar] [CrossRef]
- Dogan, M.; Silici, S.; Saraymen, R.; Ilhan, I.O. Element content of propolis from different regions of Turkey. Acta Aliment. 2006, 35, 127–130. [Google Scholar] [CrossRef]
- Roman, A.; Madras-Majewska, B.; Popiela-Pleban, E. Comparative study of selected toxic elements in propolis and honey. J. Apic. Sci. 2011, 55, 97–106. [Google Scholar]
- FAO/WHO. The 53rd Meeting of the Joint Expert Committee on Food Additives. Available online: htpp://inchem.org/documents/jecfa/jecmono/v44jec12.htm (accessed on 30 October 2015).
- Kulevanova, S.; Stafilov, T.; Dorevski, K. Determination of some macroelements in propolis by atomic absorption spectrometry. Acta Pharm. 1995, 45, 45–52. [Google Scholar]
- Shenk, J.S.; Landa, I.; Hoover, M.R.; Westerhaus, M.O. Description and Evaluation of a near Infrared Reflectance Spectro-Computer for Forage and Grain Analysis. Crop Sci. 1981, 21, 355–358. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O.; Hoover, M.R. Analysis of forages by infrared reflectance. J. Dairy Sci. 1979, 62, 807–812. [Google Scholar] [CrossRef]
- Clark, D.H.; Mayland, H.F.; Lamb, R.C. Mineral Analysis of Forages with Near Infrared Reflectance spectroscopy. Agron. J. 1987, 79, 485–490. [Google Scholar] [CrossRef]
- Ruano-Ramos, A.; Garcia-Ciudad, A.; Garcia-Criado, B. Near infrared spectroscopy prediction of mineral content in botanical fractions from semi-arids grasslands. Anim. Feed Sci. Technol. 1999, 77, 331–343. [Google Scholar] [CrossRef]
- Cozzolino, D.; Morón, A. Exploring the use of near infrared reflectance spectroscopy (NIRS) to predict trace minerals in legumes. Anim. Feed Sci. Technol. 2004, 111, 161–173. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.; Zhao, J.; Mel, H.; Wang, K.; Wang, X.; Chen, H. Determination of total flavonoids content in fresh Ginkgo biloba leaf with different colors using near infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 94, 271–276. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Martín, M.I.; Escuredo, O.; Revilla, I.; Vivar-Quintana, A.M.; Coello, M.C.; Riocerezo, C.P.; Moncada, G.W. Determination of the Mineral Composition and Toxic Element Contents of Propolis by Near Infrared Spectroscopy. Sensors 2015, 15, 27854-27868. https://doi.org/10.3390/s151127854
González-Martín MI, Escuredo O, Revilla I, Vivar-Quintana AM, Coello MC, Riocerezo CP, Moncada GW. Determination of the Mineral Composition and Toxic Element Contents of Propolis by Near Infrared Spectroscopy. Sensors. 2015; 15(11):27854-27868. https://doi.org/10.3390/s151127854
Chicago/Turabian StyleGonzález-Martín, M. Inmaculada, Olga Escuredo, Isabel Revilla, Ana M. Vivar-Quintana, M. Carmen Coello, Carlos Palacios Riocerezo, and Guillermo Wells Moncada. 2015. "Determination of the Mineral Composition and Toxic Element Contents of Propolis by Near Infrared Spectroscopy" Sensors 15, no. 11: 27854-27868. https://doi.org/10.3390/s151127854






