Soft Neurological Signs in Childhood by Measurement of Arm Movements Using Acceleration and Angular Velocity Sensors
Abstract
:1. Introduction
2. Experimental Design
2.1. System Configuration
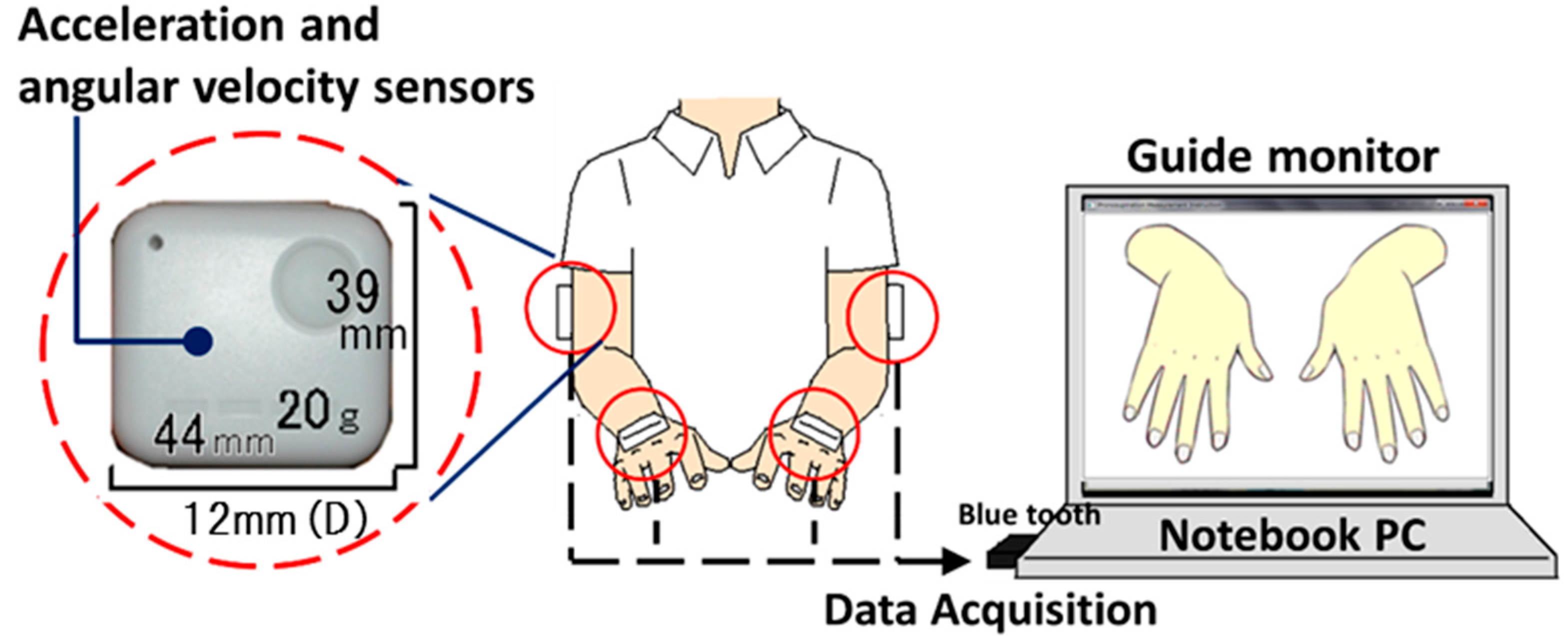
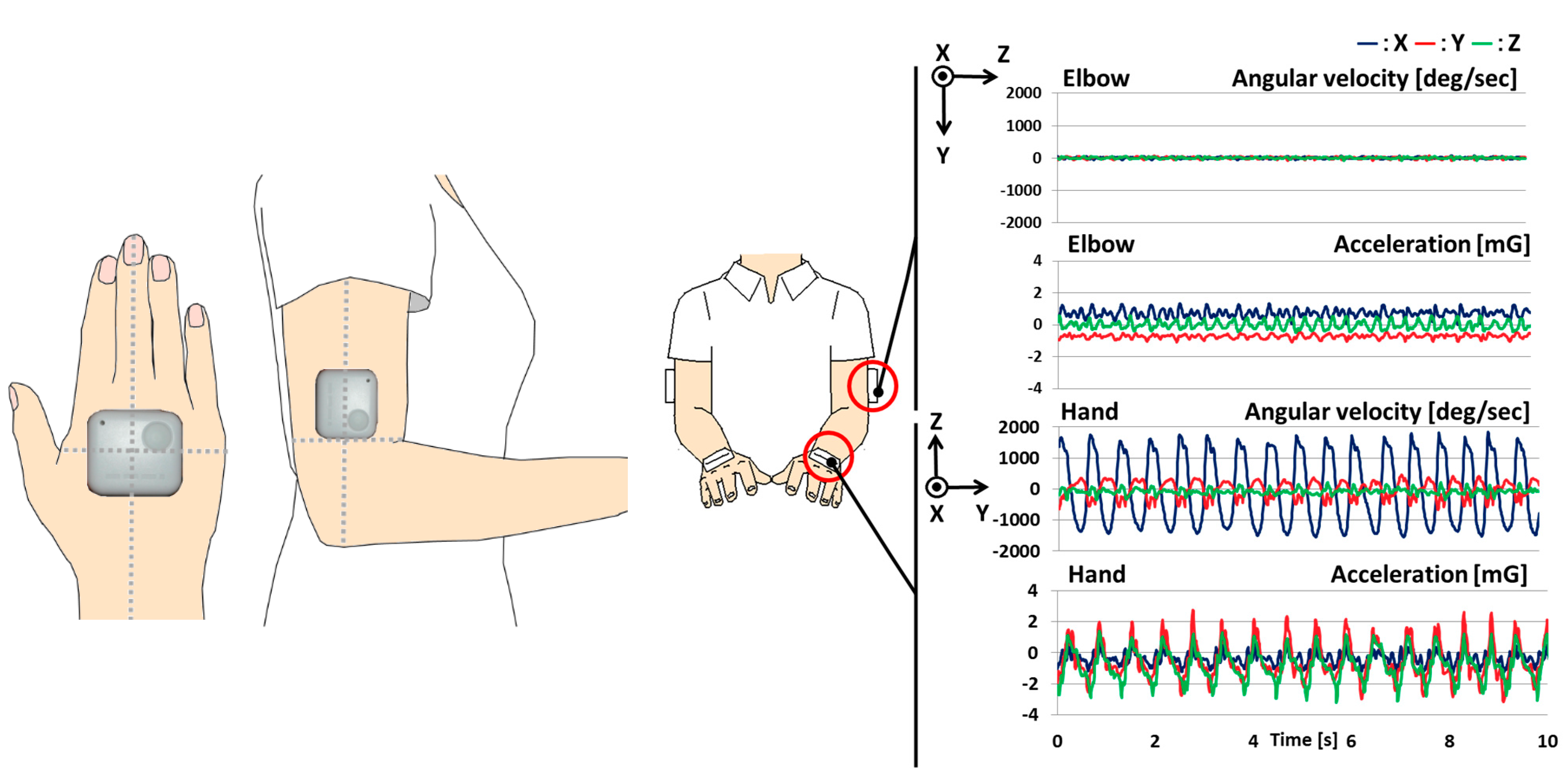
2.2. Procedure
2.3. Participants
| Age (years) | Male | Female | Total |
|---|---|---|---|
| 4 | 6 | 3 | 9 |
| 5 | 9 | 5 | 14 |
| 6 | 4 | 7 | 11 |
| 7 | 13 | 19 | 32 |
| 8 | 22 | 14 | 36 |
| 9 | 19 | 21 | 40 |
| 10 | 17 | 20 | 37 |
| 11 | 8 | 18 | 26 |
| 12 | 9 | 9 | 18 |
| 21–26 | 16 | 2 | 18 |
| Total | 123 | 118 | 241 |
3. Analysis Design
3.1. Evaluation Indices
Analyzed Parameters
3.2. Evaluation Score and Statistics
4. Results
4.1. Development Curves for the Imitative Motor Task
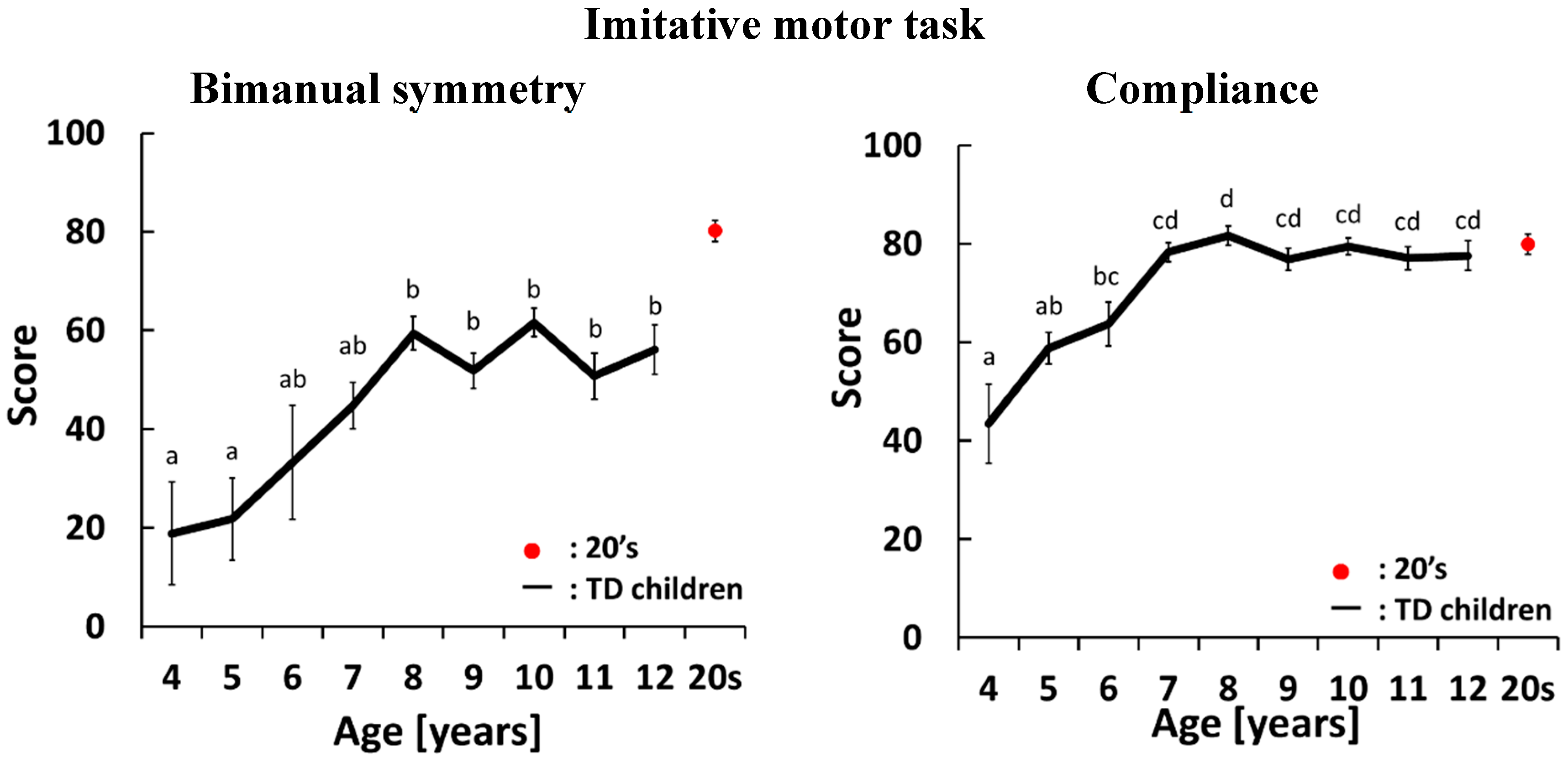
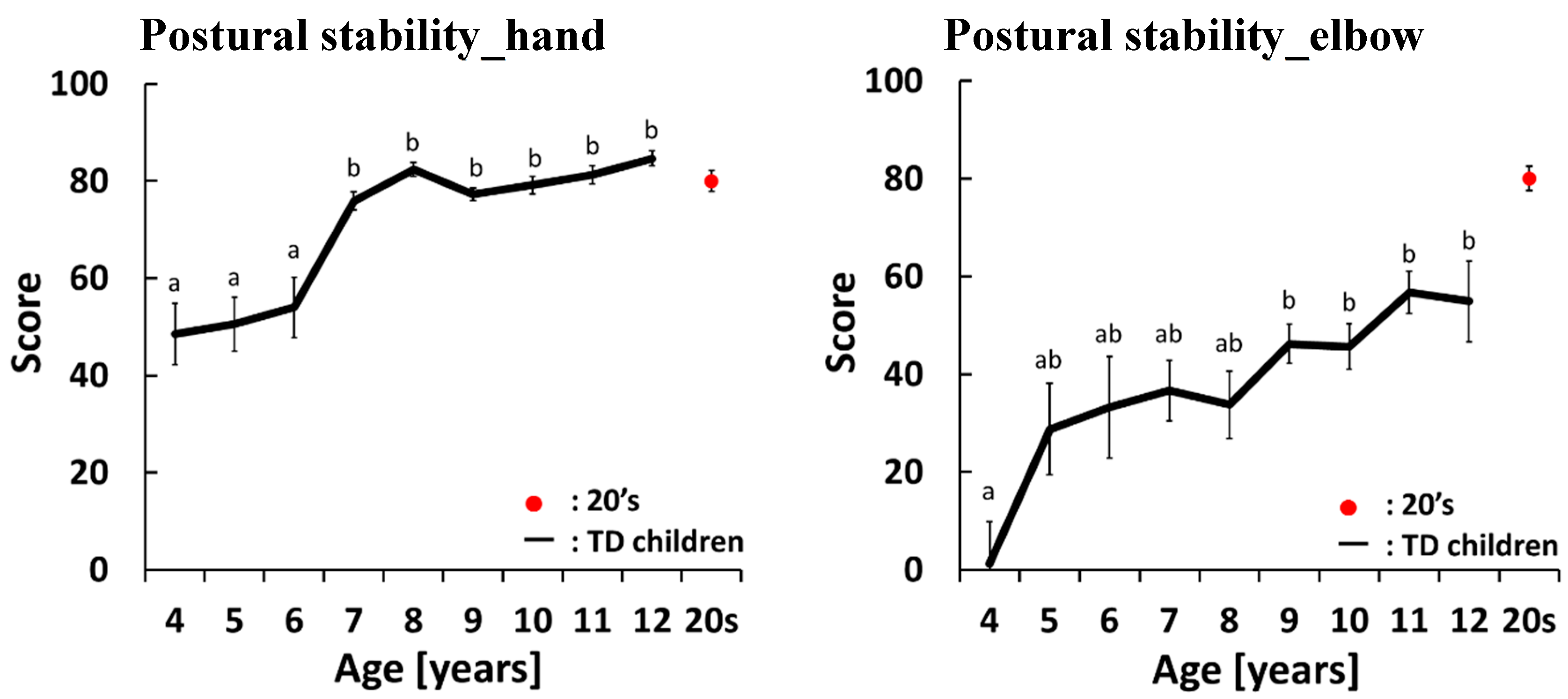
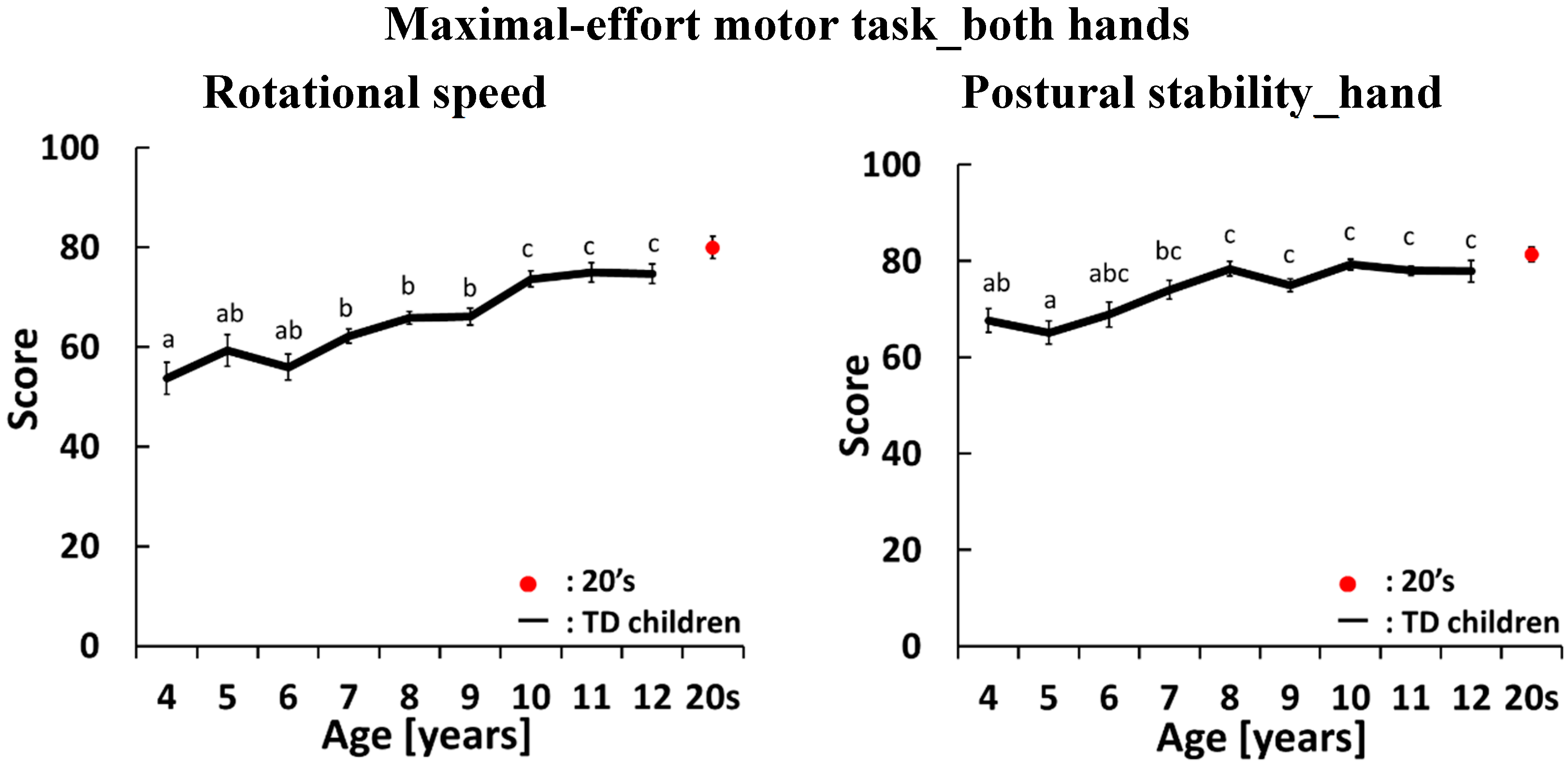
4.2. Development Curves for the Maximal-Effort motor Task Performed with Both Hands

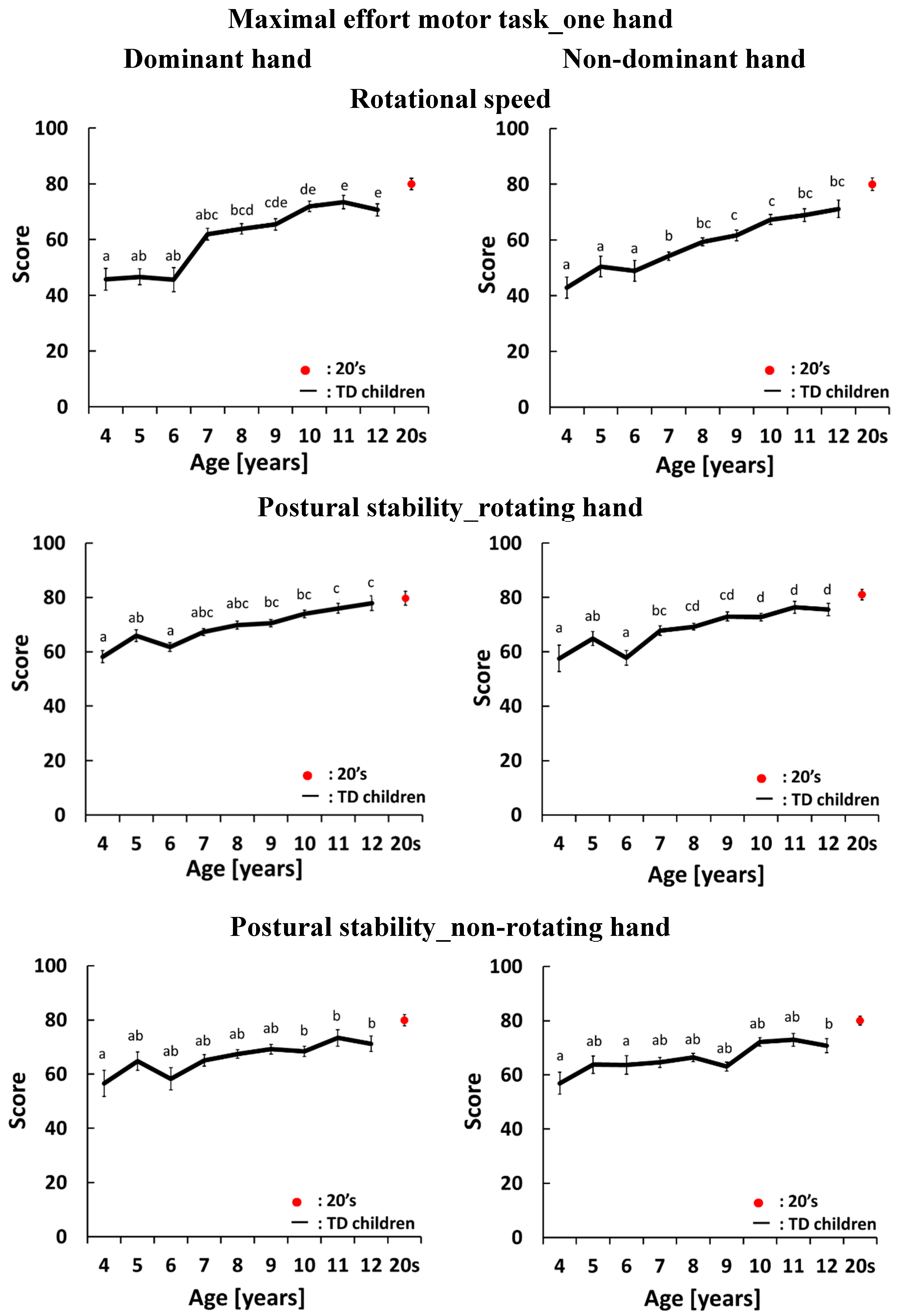
4.3. Development Curves for the Maximal-Effort Motor Task Performed with One Hand

4.4. Radar Charts of Pronation and Supination


5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Isabel, P.M.; Martin, L.; Henrique, L.; Helena, A.; Gail, R.; Peter, D.S.; Brenda, D.T. Neurological Subtle Signs and cognitive development: A study in late childhood and adolescence. Child Neuropsychol. 2013, 19, 466–478. [Google Scholar]
- Largo, R.H.; Fischer, J.E.; Rousson, V. Neuromotor development from kindergarten age to adolescence: Developmental course and variability. Swiss Med. Wkly. 2003, 133, 193–199. [Google Scholar] [PubMed]
- Largo, R.H.; Caflish, J.A.; Hug, F.; Muggli, K.; Monar, A.A. Neuromotor development from 5 to 18 years. Part1: Timed performance. Dev. Med. Child Neurol. 2001, 43, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.H.; Caflish, J.A.; Hug, F.; Muggli, K.; Monar, A.A. Neuromotor development from 5 to 18 years. Part 2: Associated movements. Dev. Med. Child Neurol. 2001, 43, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Fellick, J.M.; Thomson, A.P.; Sills, J.; Hart, C.A. Neurological soft signs in mainstream pupils. Arch. Dis. Child. 2001, 85, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Iannetti, P.; Di, N.S.; Mastrangelo, M. Neurological “soft signs” in children and adolescents. J. Pediatr. Neurol. 2005, 3, 123–125. [Google Scholar]
- Gasser, T.; Rousson, V.; Caflisch, J.; Remo, L. Quantitative reference curves for associated movements in children and adolescents. Dev. Med. Child Neurol. 2007, 49, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Isabel, M. A longitudinal study of neurological soft signs from late childhood into early adulthood. Dev. Med. 2008, 50, 602–607. [Google Scholar]
- Rueckriegel, S.M.; Blankenburg, F.; Burghardt, R.; Ehrlich, S.; Henze, G.; Mergl, R.; Hernáiz, D.P. Influence of age and movement complexity on kinematic hand movement parameters in childhood and adolescence. Int. J. Dev. Neurosci. 2008, 26, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.; Rousson, M.; Caflisch, J.; Jenni, O.G. Development of motor speed and associated movements from 5 to 18 years. Dev. Med. Child Neurol. 2010, 52, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Bhoomika, R.K.; Shobini, L.R.; Thennarasu, K. Growth patterns of neuropsychological functions in Indian children. Front. Psychol. 2011, 2, 1–15. [Google Scholar]
- Kakebeeke, T.H.; Caflisch, J.; Chaouch, A.; Rousson, V.; Largo, R.H.; Jenni, O.G. Neuromotor development in children. Part 3: Motor performance in 3- to 5-year-olds. Dev. Med. Child Neurol. 2013, 55, 248–256. [Google Scholar] [CrossRef]
- Mijna, H.A. The Neurological Examination of the Child with Minor neurological Dysfunction, 3rd ed.; Mac Keith Press: London, UK, 2010; pp. 61–75. [Google Scholar]
- Benjamin, C.C.; Massimo, C.; Alberto, J.E. Mirror Movement in Movement Disorders: A Review. Tremor Other Hyperkinet. Mov. 2012, 2, 1–7. [Google Scholar]
- Kreulen, M.; Smeulders, M.J.C.; Veeger, H.E.J.; Hage, J.J.; Vanderhorst, C.M.M. Three-dimensional video analysis of forearm rotation before and after combined pronator teres rerouting and flexor carpi ulnaris tendon transfer surgery in patients with cerebral palsy. J. Hand Surg. Br. Eur. 2004, 29, 55–60. [Google Scholar] [CrossRef]
- Okada, M.; Okada, M. A method for quantification of alternate pronation and supination for forearms. Comput. Biomed. Res. 1983, 16, 59–78. [Google Scholar] [CrossRef]
- Hermsdorfer, J. Comparative analysis of diadochokinetic movements. J. Electromyogr. Kinesiol. 1999, 9, 283–295. [Google Scholar] [CrossRef]
- Iramina, K.; Kamei, Y.; Katayama, Y. Evaluation System for Minor Nervous Dysfunction by Pronation and Supination of Forearm using Wireless Acceleration and Angular Velocity Sensors. In Proceedings of the IEEE EMBC, Boston, MA, USA, 30 August–3 September 2011; pp. 7364–7367.
- Shah, H.; Kamath, R.; Patankar, V.; Sangle, J.; Dave, M. Neurological soft signs in children with attention deficit hyperactivity disorder. Indian J. Psychiatry 2012, 54, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Touwen, B.C.L.; Prechtl, H.F.R. The Neurological Examination of the Child with Minor Neurological Dysfunction; William Heinemann Medical Books: London, UK, 1970; pp. 42–44. [Google Scholar]
- Carl, G.; Priscial, C.; Tatiana, B. Examing age-related movement representations for sequential (fine-motor) finger movenets. Brain Cognit. 2011, 77, 459–463. [Google Scholar]
- Flatters, I.; Hill, L.J.; Liam, J.B.; Williams, J.H.G.; Barber, S.E.; Mon, W.M. Manual Control Age and Sex Differences in 4 to 11 Year Old Children. PLoS ONE 2014, 9, 1–12. [Google Scholar]
- Hadders, A.M.; Touwen, B.C.L.; Olinga, A.A. Minor neurological dysfunction and behavioural development. A report from the Groningen Perinatal Project. Early Hum. Dev. 1985, 11, 221–229. [Google Scholar] [CrossRef]
- Van Mier, H. Developmental differences in drawing performance of the dominant and non-dominant hand in right-handed boys and girls. Hum. Mov. Sci. 2006, 25, 657–677. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneko, M.; Yamashita, Y.; Inomoto, O.; Iramina, K. Soft Neurological Signs in Childhood by Measurement of Arm Movements Using Acceleration and Angular Velocity Sensors. Sensors 2015, 15, 25793-25808. https://doi.org/10.3390/s151025793
Kaneko M, Yamashita Y, Inomoto O, Iramina K. Soft Neurological Signs in Childhood by Measurement of Arm Movements Using Acceleration and Angular Velocity Sensors. Sensors. 2015; 15(10):25793-25808. https://doi.org/10.3390/s151025793
Chicago/Turabian StyleKaneko, Miki, Yushiro Yamashita, Osamu Inomoto, and Keiji Iramina. 2015. "Soft Neurological Signs in Childhood by Measurement of Arm Movements Using Acceleration and Angular Velocity Sensors" Sensors 15, no. 10: 25793-25808. https://doi.org/10.3390/s151025793





