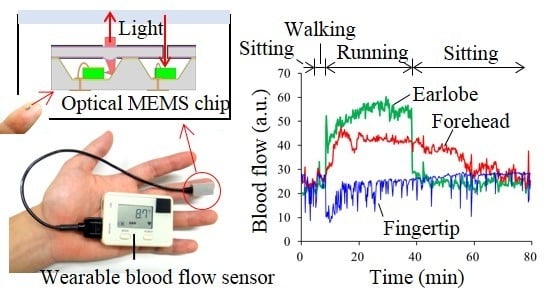
Detection of Site-Specific Blood Flow Variation in Humans during Running by a Wearable Laser Doppler Flowmeter
Abstract
:1. Introduction
2. Experimental Section
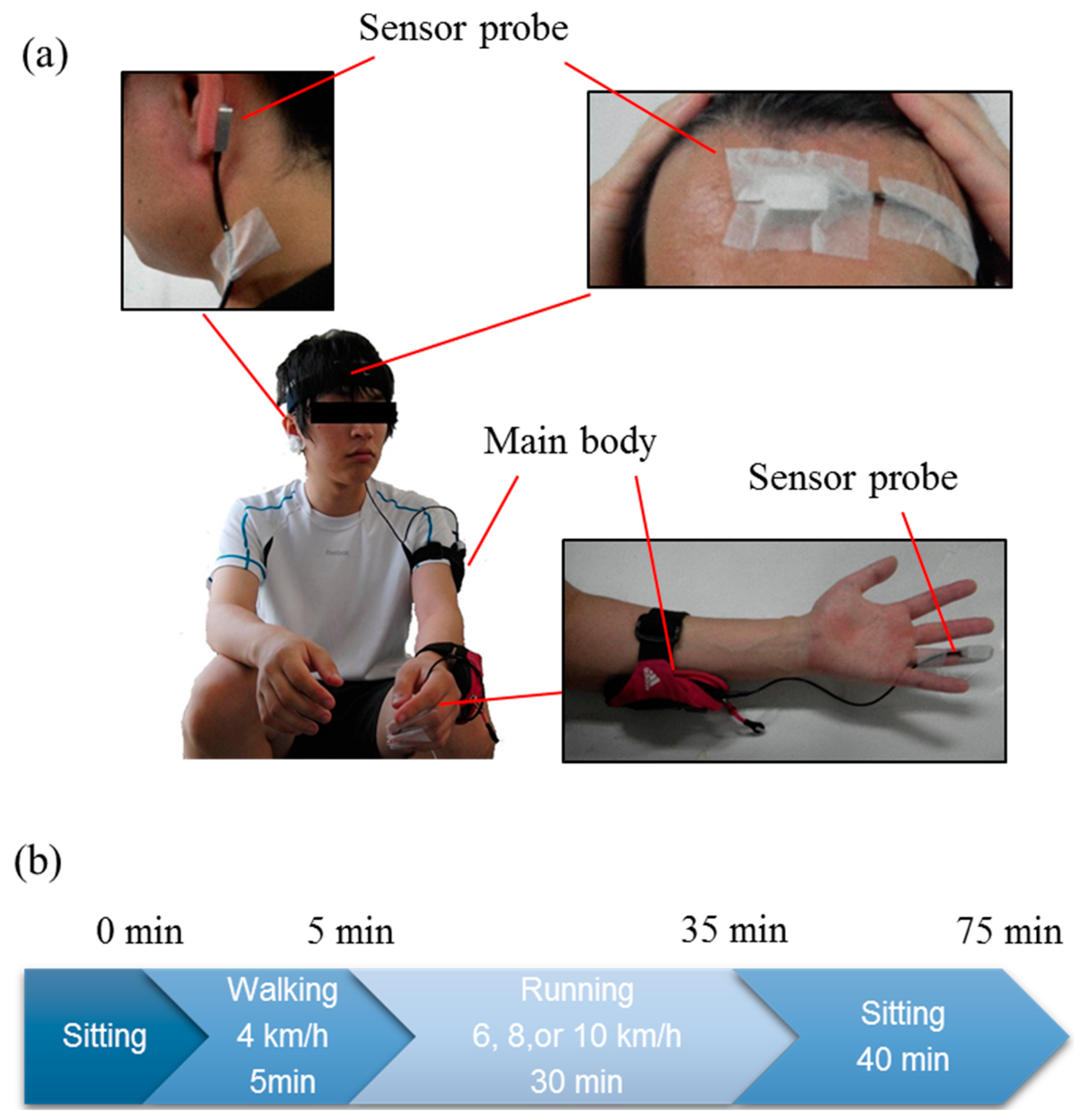
2.1. Subjects
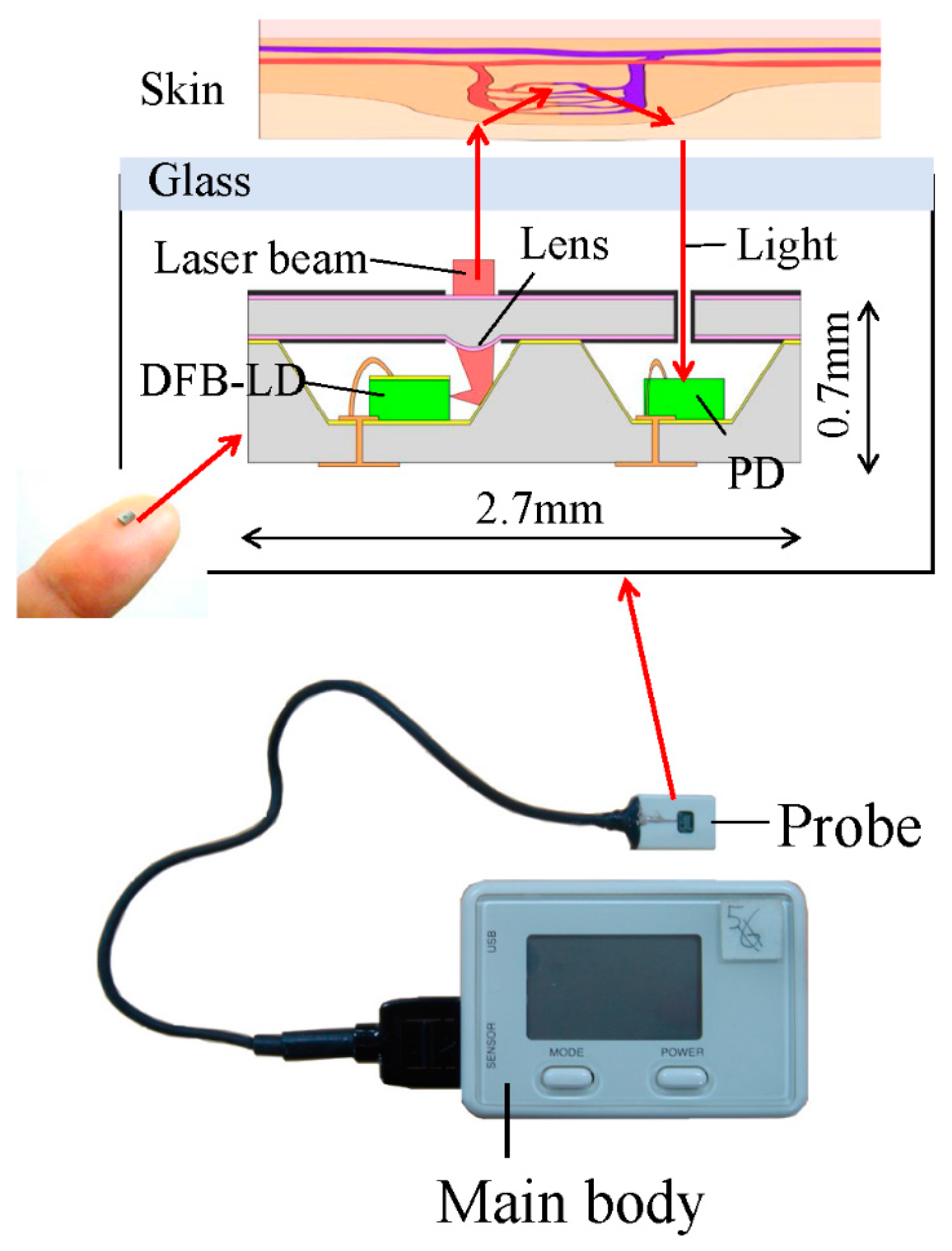
2.2. MEMS Blood Flow Sensor

2.3. Experimental Designs
2.4. Analysis
3. Results
3.1. Stability of Blood Flow Signals at Each Measurement Site
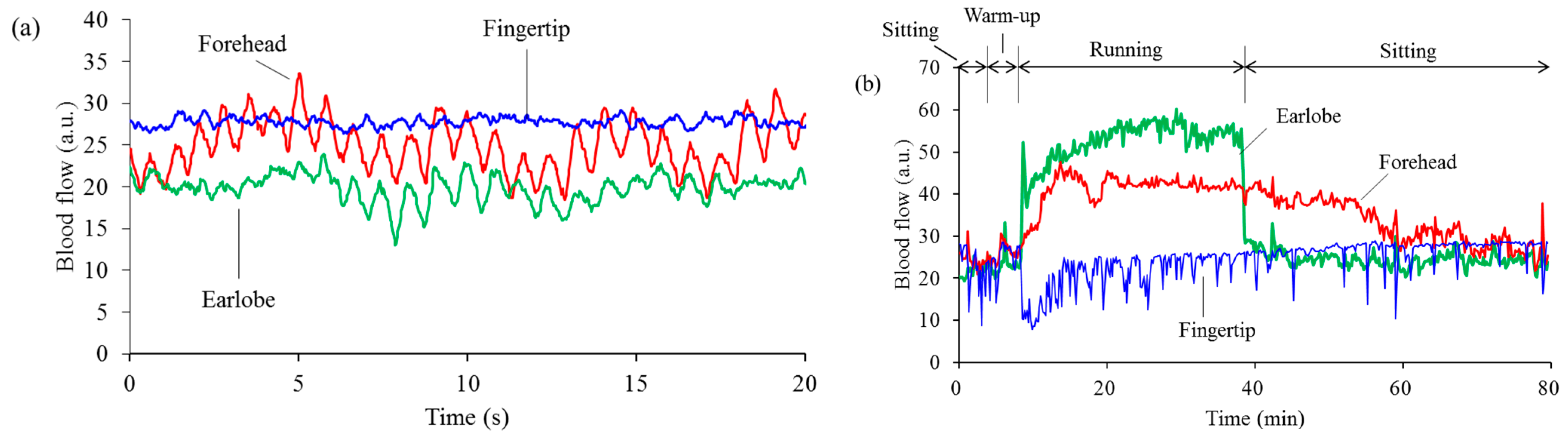
3.2. Separation of Heartbeat from Heart Rhythm while Running
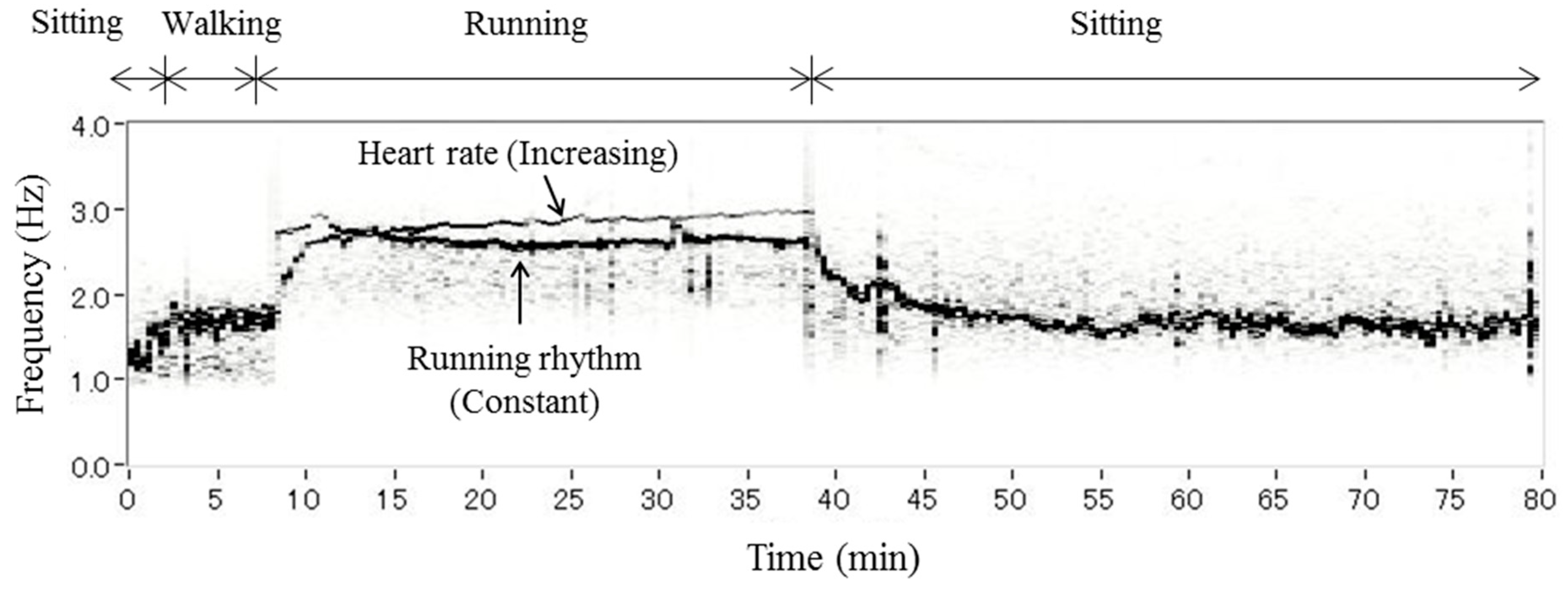
3.3. Differences among Running Speeds
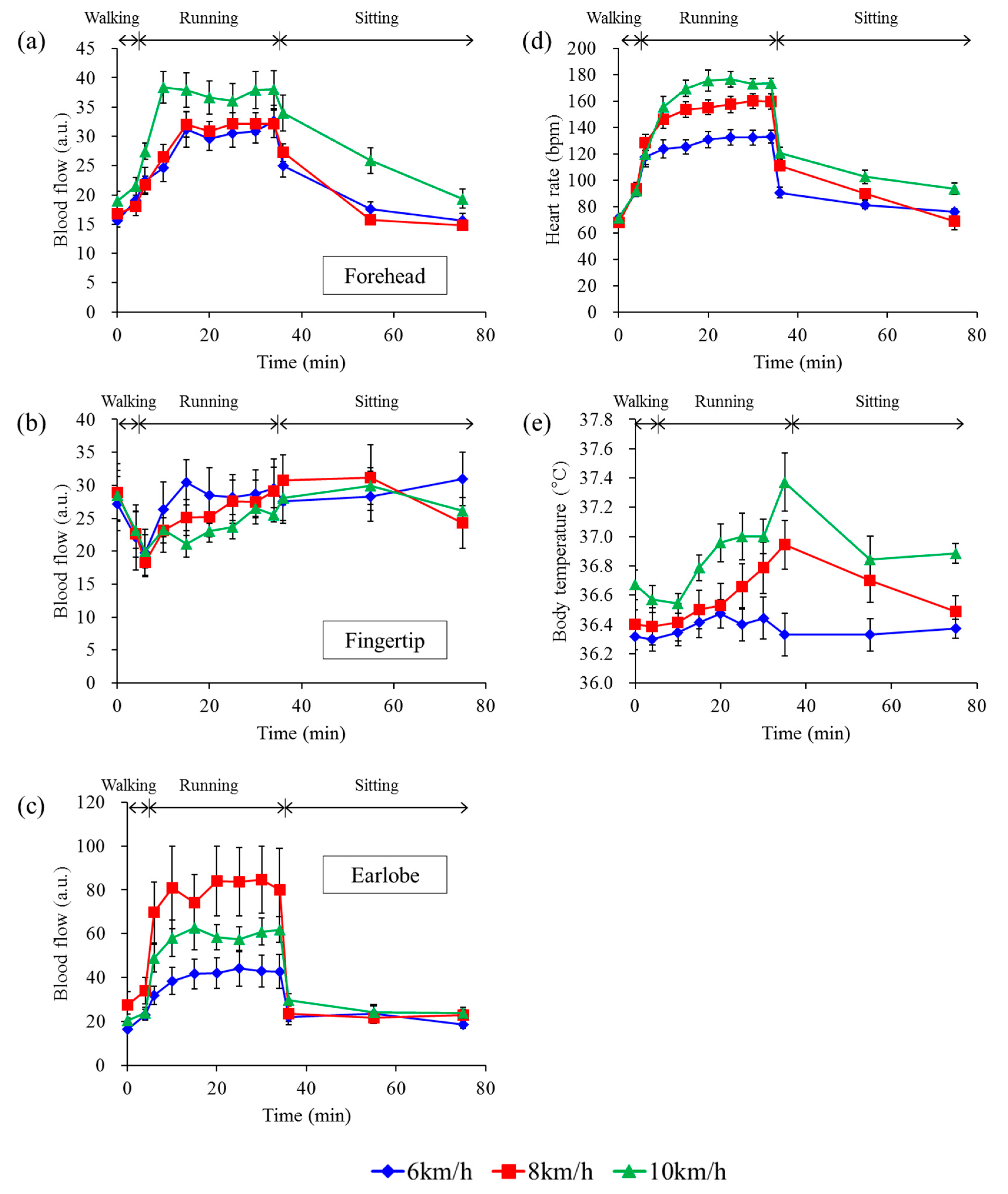
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Itao, K.; Umeda, T.; Loipez, G.; Kinjo, M. Human recorder system development for sensing the autonomic nervous system. In Proceedings of the IEEE Sensors 2008, Lecce, France, 26–29 October 2008; pp. 423–426.
- Zhu, Z.; Liu, T.; Li, G.; Li, T.; Inoue, Y. Wearable sensor systems for infants. Sensors 2015, 15, 3721–3749. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chan, M.C.; Chen, C.Y.; Lin, B.S. Novel wearable and wireless ring-type pulse oximeter with multi-detectors. Sensors 2014, 14, 17586–17599. [Google Scholar] [CrossRef] [PubMed]
- Ur Rehman, M.H.; Liew, C.S.; Wah, T.Y.; Shuja, J.; Daghighi, B. Mining personal data using smartphones and wearable devices: A survey. Sensors 2015, 15, 4430–4469. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Hamida, E.B. Surveying wearable human assistive technology for life and safety critical applications: Standards, challenges and opportunities. Sensors 2014, 14, 9153–9209. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.L., Jr.; Johnson, W.L.; Kenney, W.L.; Pergola, P.E.; Kosiba, W.A. Mechanisms of control of skin blood flow during prolonged exercise in humans. Am. J. Physiol. 1993, 265, H562–H568. [Google Scholar] [PubMed]
- Yasuda, Y.; Yoshizawa, M.; Nishino, H. Effect of Exercise Intensity on the Spectral Properties of Skin Blood Flow. Jpn. J. Physiol. 1994, 44, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.G.; Galbet, J.A.L.; Nielsen, B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J. Physiol. 1998, 513, 895–905. [Google Scholar] [CrossRef]
- Roustit, M.; Blaise, S.; Millet, C.; Cracowski, J.L. Reproducibility and methodological issue of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetery. Microvasc. Res. 2010, 79, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Tew, G.A.; Klonizakis, M.; Crank, H.; Briers, J.D.; Hodges, G.J. Comparison of laser speckle contrast imaging with laser Doppler for assessing microvascular function. Microvasc. Res. 2011, 82, 326–332. [Google Scholar] [Green Version]
- Roustit, M.; Cracowski, J.L. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol. Sci. 2013, 34, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kvandal, P.; Landsverk, S.A.; Bernjak, A.; Stefanovska, A.; Kvernmo, H.D.; Kirkebøen, K.A. Low-frequency oscillations of the laser Doppler perfusion signal in human skin. Microvasc. Res. 2006, 72, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kano, T.; Shimoda, O.; Higashi, K.; Sadanaga, M. Fundamental patterns and characteristics of the laser-Doppler skin blood flow waves recorded from the finger or toe. J. Auton. Nerv. Syst. 1993, 45, 191–199. [Google Scholar] [CrossRef]
- Sasano, H.; Hayano, J.; Tsuda, T.; Katsuya, H. Effects of sympathetic nerve blockades on low-frequency oscillations of human earlobe skin blood flow. J. Auton. Nerv. Syst. 1999, 77, 60–67. [Google Scholar] [CrossRef]
- Mukae, H.; Mochizuki, M.; Taniguchi, Y. Physiological Analysis of Mood State Using Spontaneous Rhythmic Oscillations Measured with Laser Doppler Flowmetry. Jpn. J. Physiol. Anthropol. 2006, 11, 81–86. (In Japanese) [Google Scholar]
- Johnson, J.M.; Proppe, D.W. Cardiovascular adjustments to heat stress. In Handbook of Physiology; Oxford University Press: New York, NY, USA, 1996; pp. 215–243. [Google Scholar]
- Lossius, K.; Eriksen, M.; Walloe, L. Fluctuations in blood flow to acral skin in humans: Connection with heart rate and blood pressure variability. J. Physiol. 1993, 460, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Miyazaki, T.; Ito, H.; Nogami, H.; Higurashi, E.; Ando, S.; Sawada, R. Comparable accuracy of micro-electromechanical blood flowmetry based analysis vs. electrocardiography-based analysis in evaluating heart rate variability. Jpn. Circ. J. 2015, 79, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Holloway, G.A., Jr. An instrument to measure cutaneous blood flow using the Doppler shift of laser light. IEEE Trans. Biomed. Eng. 1978, BME-25, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, R.W.; Folger, R.L.; Giddon, D.B.; Ware, B.R. Laser Doppler blood flow meter and optical plethysmograph. Rev. Sci. Instrum. 1980, 51, 169–172. [Google Scholar] [CrossRef]
- Newson, T.P.; Obeid, A.; Wolton, R.S.; Boggett, D.; Rolfe, P. Laser Doppler velocimetry: The problem of fiber movement artefact. J. Biomed. Eng. 1987, 9, 169–172. [Google Scholar] [CrossRef]
- De Mul, F.F.M.; van Spijker, J.; van der Plas, D.; Greve, J.; Aarnoudse, G.; Smits, T.M. Mini laser-Doppler (blood) flow monitor with diode laser source and detection integrated in the probe. Appl. Opt. 1984, 23, 2970–2973. [Google Scholar] [CrossRef] [PubMed]
- Higurashi, E.; Sawada, R.; Ito, T. An integrated laser Doppler blood flowmeter. J. Lightwave Technol. 2003, 21, 591–595. [Google Scholar] [CrossRef]
- Kimura, Y.; Goma, M.; Onoe, A.; Higurashi, E.; Sawada, R. Integrated Laser Doppler Blood Flowmeter Designed to Enable Wafer-Level Packaging. IEEE Trans. Biomed. Eng. 2010, 57, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, W.; Nogami, H.; Higurashi, E.; Sawada, R. Miniaturization of a laser Doppler blood flow sensor by system-in-package technology: Fusion of an optical microelectromechanical system chip and integrated circuits. IEEJ Trans. Electr. Electron. Eng. 2010, 5, 137–142. [Google Scholar] [CrossRef]
- Nogami, H.; Iwasaki, W.; Abe, T.; Kimura, Y.; Onoe, A.; Higurashi, E.; Takeuchi, S.; Kido, M.; Furue, M.; Sawada, R. Use of a simple arm-raising test with a portable laser Doppler blood flow meter to detect dehydration. Proc. Inst. Mech. Eng. Part H: J. Eng. Med. 2011, 225, 411–419. [Google Scholar] [CrossRef]
- Iwasaki, W.; Nogami, H.; Ito, H.; Gotanda, T.; Peng, Y.; Takeuchi, S.; Furue, M.; Higurashi, E.; Sawada, R. Useful Method to Monitor the Physiological Effects of Alcohol Ingestion by Combination of Micro-integrated Laser Doppler Blood Flow Meter and Arm-Raising Test. Proc. Inst. Mech. Eng. Part H: J. Eng. Med. 2012, 226, 759–765. [Google Scholar] [CrossRef]
- Kido, M.; Takeuchi, S.; Hayashida, S.; Urabe, K.; Sawada, R.; Furue, M. Assessment of abnormal blood flow and efficacy of treatment in patients with systemic sclerosis using a newly developed micro wireless laser Doppler flowmeter and arm-raising test. Br. J. Drmatol. 2007, 157, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, K.; Iwasaki, W.; Nakamura, M.; Higurashi, E.; Soh, T.; Itho, T.; Okada, H.; Maeda, R.; Sawada, R. Development of a wireless sensor for the measurement of chicken blood flow using the laser Doppler blood flow meter technique. IEEE Trans. Biomed. Eng. 2013, 60, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Bonner, R.; Nossal, R. Model for laser Doppler Measurements of blood flow in tissue. Appl. Opt. 1981, 20, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S516. [Google Scholar] [CrossRef] [PubMed]
- Jette, M.; Sidney, K.; Blumchen, G. Metabolic Equivalents (METS) in Exercise Testing, Exercise Prescription, and Evaluation of Functional Capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- McArdle, W.; Katch, F.; Katch, V. Exercise Physiology: Energy Nutrition, and Human Performance; Lea & Febiger: Philadelphia, PA, USA, 1986; p. 138. [Google Scholar]
- Charkoudian, N. Skin Blood Flow in Adult Human Thermoregulation: How It Works, When It Does Not, and Why. Mayo Clin. Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Kurozawa, Y.; Ohshiro, H.; Iwai, N.; Meshitsuka, S.; Nose, T. Heart rate, blood pressure and forearm skin blood flow during exercise test in different ambient temperatures. J. Yonag Med. Ass. 1991, 42, 262–268. (In Japanese) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, W.; Nogami, H.; Takeuchi, S.; Furue, M.; Higurashi, E.; Sawada, R. Detection of Site-Specific Blood Flow Variation in Humans during Running by a Wearable Laser Doppler Flowmeter. Sensors 2015, 15, 25507-25519. https://doi.org/10.3390/s151025507
Iwasaki W, Nogami H, Takeuchi S, Furue M, Higurashi E, Sawada R. Detection of Site-Specific Blood Flow Variation in Humans during Running by a Wearable Laser Doppler Flowmeter. Sensors. 2015; 15(10):25507-25519. https://doi.org/10.3390/s151025507
Chicago/Turabian StyleIwasaki, Wataru, Hirofumi Nogami, Satoshi Takeuchi, Masutaka Furue, Eiji Higurashi, and Renshi Sawada. 2015. "Detection of Site-Specific Blood Flow Variation in Humans during Running by a Wearable Laser Doppler Flowmeter" Sensors 15, no. 10: 25507-25519. https://doi.org/10.3390/s151025507