One Single Molecule as a Multifunctional Fluorescent Probe for Ratiometric Sensing of Fe3+, Cr3+ and Colorimetric Sensing of Cu2+
Abstract
: The reagent Rh-C, incorporating a rhodamine moiety and a coumarin backbone and prepared via click chemistry, was developed as the first single molecule for detecting Cu2+, Fe3+ and Cr3+. Its response to Cu2+ in different solutions is visible to the naked eye and it exhibits a ratiometric fluorescence response to Fe3+ in methanol and Cr3+ in acetonitrile.1. Introduction
Metal ions play very important roles in living system and have significant impacts on human health. Cu2+, Fe3+ and Cr3+ are indispensable nutrients for life, and both their deficiency and excesses are connected to various disorders [1–3]. For instance, during the uptake, storage and trafficking of Cu2+, a deficiency is proposed to be associated with severe diseases such as Menkes syndrome, Wilson's disease and Alzheimer's disease [4–6]. Fe3+ is one of the most essential metals in oxygen transport and electron transport [2,7,8]. Fe3+deficiency in our bodies will lead to low oxygen delivery, which could result in low blood pressure, anemia and decreased immunity [9,10]. On the contrary, excess Fe3+ can result in the formation of reactive oxygen species (ROS), which have damaging effects on lipids, nucleic acids, and proteins [11,12]. A normal person needs to absorb 25–35 mg of Cr3+ per day to improve the metabolism of glucose and lipids [13], while excessive intake of Cr3+ has an adverse effect on cellular structure [14,15]. In consideration of the necessity and toxicity of Cu2+, Fe3+ and Cr3+, the design of new probes for exact and convenient detection of Cu2+, Fe3+ and Cr3+ in environmental and biological samples is of much interest.
In recent years, because of the inexpensiveness, high sensitivity and simplicity of fluorescence analysis [16], fluorescent indicators have attracted widespread attention, and a rapidly increasing number of metal-responsive fluorescent sensors have been studied [17–19]. Although chemists have developed a great deal of fluorescent probes for Cu2+ [20–23], Fe3+ [24] and Cr3+ [25–30], respectively, probes which can indicate Cu2+, Fe3+ and Cr3+ at the same time via a single molecular species have not been reported. Herein, we intend to develop a fluorescent probe that could distinguish Cu2+, Fe3+ and Cr3+ under different conditions with one single molecule.
According to previous studies of other researchers, rhodamine dyes are generally used as fluorescent probes because of their excellent photostability, photophysical properties and suitable water-solubility [31,32]. On the other hand, coumarin, as another well-known strongly fluorescent compound, is easy and convenient to synthesize in general [33]. To date, some FRET probes based on rhodamine and coumarin to achieve ratiometric fluorescent responses have been reported [34–36]. Inspired by these works, we sought to design a FRET ratiometric fluorescent probe Rh-C by connecting the rhodamine B and coumarin moieties with a 1,2,3-triazole linker (Scheme 1), in the hope that the introduction of the triazole system might provide an additional coordination site [37–39]. Rh-C and the intermediates were characterized by 1H-NMR, 13C-NMR, MS (see Supporting Information).
2. Results and Discussion
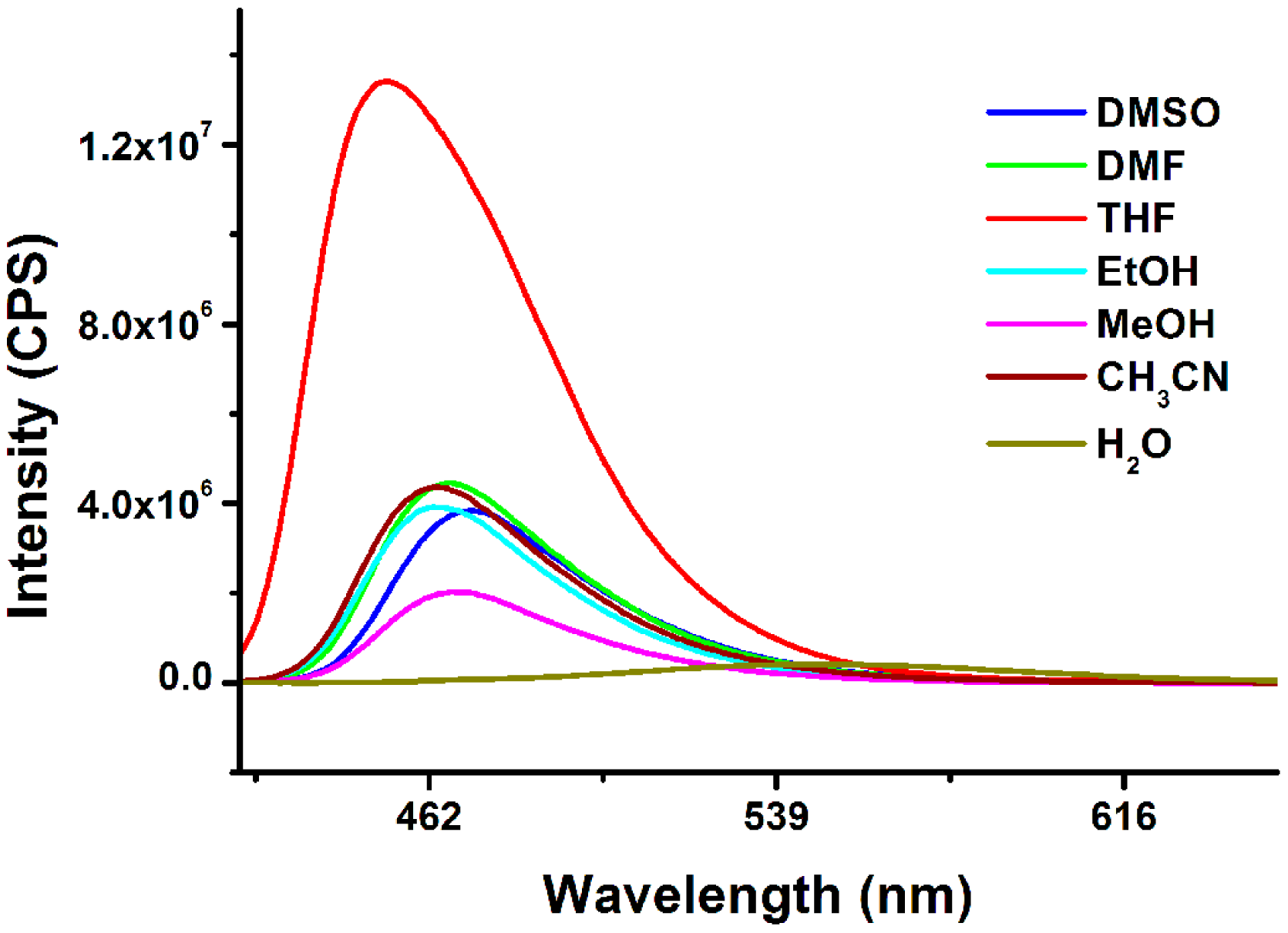
Firstly, the fluorescence emission profiles of Rh-C in different solvents (DMSO, DMF, THF, EtOH, MeOH, MeCN, H2O) were studied. As shown in Figure 1, the emission peak of Rh-C at ∼470 nm which represented the characteristic peak of coumarin was almost unchanged in different organic solvents. Meanwhile, no peak was found at ∼580 nm corresponding to the emission of rhodamine B, indicating that the spirolactam of rhodamine moiety remained closed [31,32]. Surprisingly, the fluorescence of Rh-C was fairly weak and the emission peak was obviously red-shifted in water, which might be due to the TICT property of the 7-diethylamino group of the coumarin moiety [40]. Thus, the properties of Rh-C were mainly studied in organic solutions.
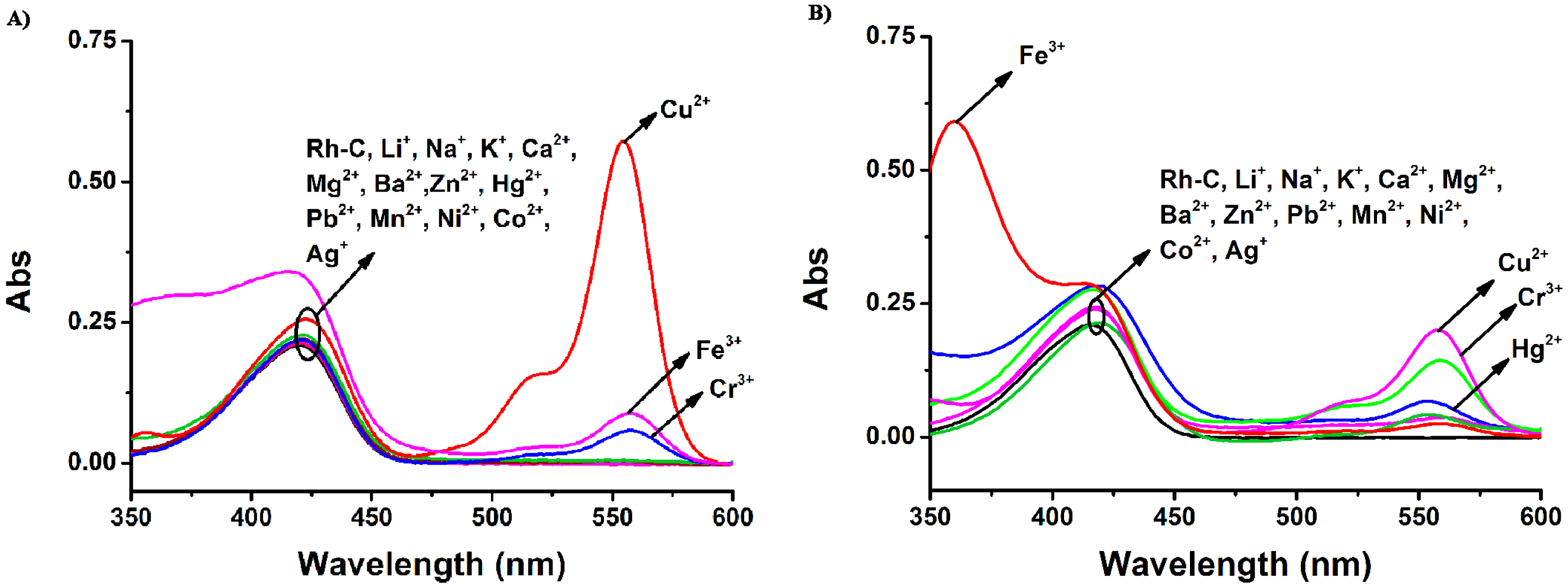
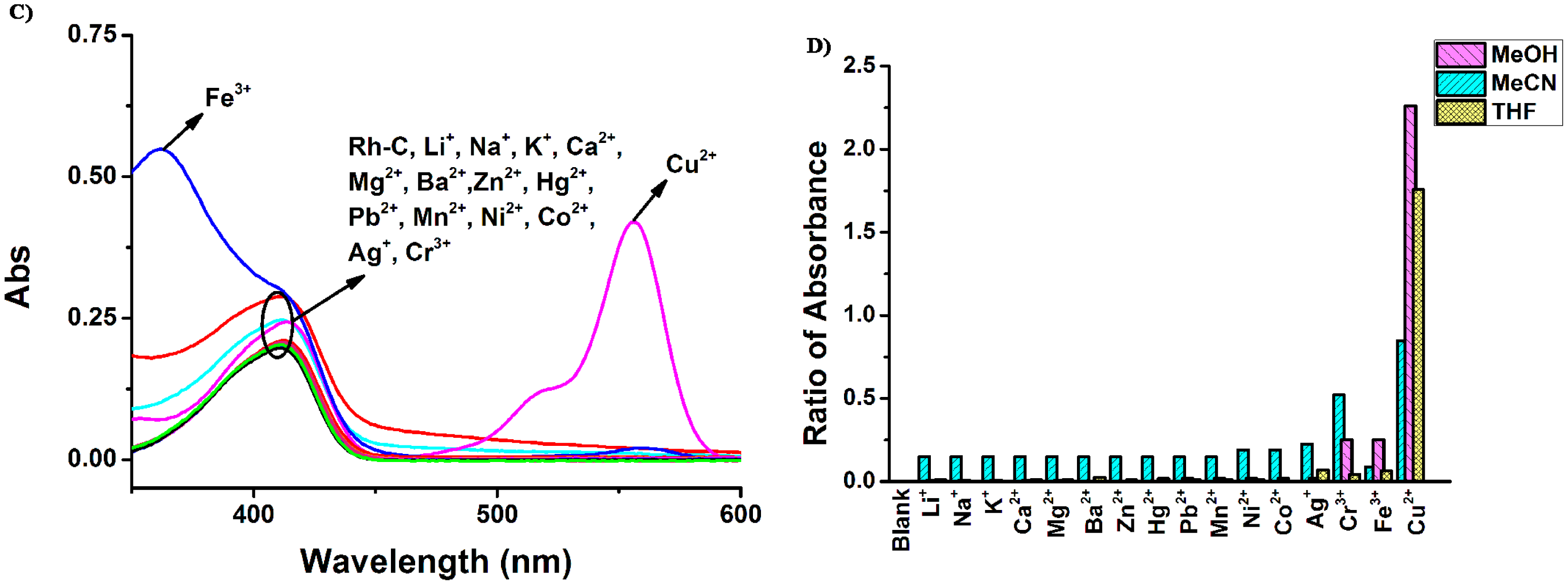
Then, the cation selectivity of Rh-C in different solvents was investigated through UV-Vis absorption and fluorescence emission spectroscopy. As illustrated in Figure 2, the absorption peaks of Rh-C (5 μM) were located at around 415 nm in MeOH, MeCN and THF and the addition of 20 equiv of a range of physiologically and environmentally relevant metal ions (Li+, Na+, K+, Ca2+, Mg2+, Ba2+, Zn2+, Hg2+, Pb2+, Mn2+, Ni2+, Co2+ and Ag+) did not cause any significant changes in the absorption spectra. However, in MeOH solution, the addition of 20 equiv of Cr3+, Fe3+ and Cu2+ led to the appearance of a typical peak at 550 nm related to the opening of the spiro ring of rhodamine B (for Cr3+ 11-fold, for Fe3+ 17-fold, and for Cu2+ 114-fold), accompanied by a color change from light yellow to pink. In MeCN solution of Rh-C, the addition of Cu2+ and Cr3+ could cause a slight enhancement of the absorption at 558 nm, but only Cu2+ could induce a color change from light yellow to pink. In THF solution of Rh-C, only the addition of Cu2+ could cause a remarkable increase of absorption at ∼550 nm, implying that Rh-C exhibited an excellent selectivity towards Cu2+ in THF.
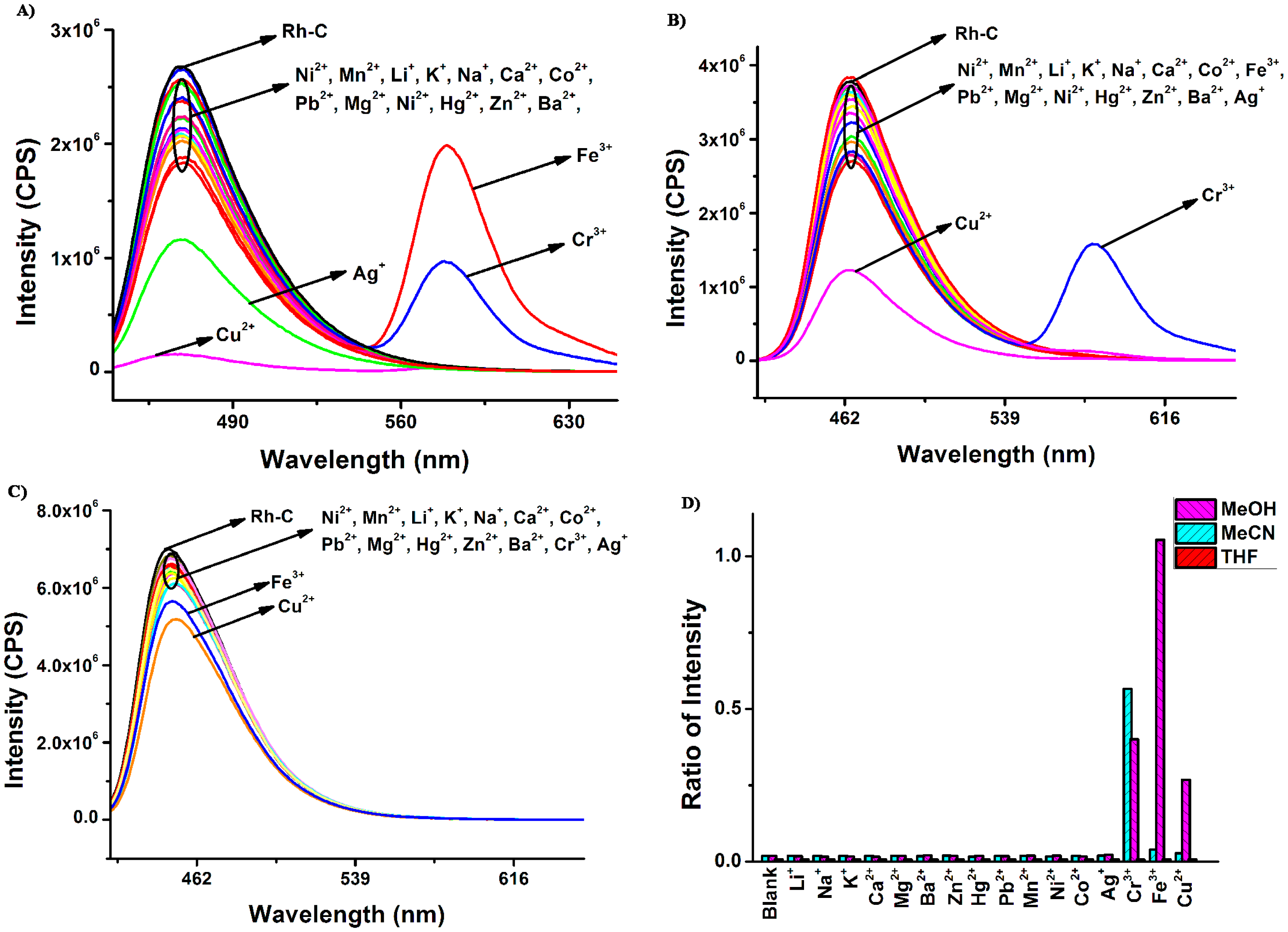
To sum up, Rh-C exhibited selective colorimetric response towards Cu2+ in different organic solution, particularly in THF. The fluorescence responses of Rh-C (5 μM) towards various metal ions are shown in Figure 3.
As envisioned, in MeOH, MeCN and THF, there were no significant changes in the fluorescence spectra after the addition of 20 equiv of Li+, Na+, K+, Ca2+, Mg2+, Ba2+, Zn2+, Hg2+, Pb2+, Mn2+, Ni2+ and Co2+, but surprisingly, the addition of Cu2+ did not result in the appearance of the typical emission peak of rhodamine B at ∼580 nm but rather led to more or less fluorescence quenching at ∼470 nm in all solvents, while the spiro ring opening of rhodamine B indeed occurred in the presence of Cu2+. We assumed that although the coordination between Cu2+ with Rh-C caused the spiro ring opening of rhodamine B, the fluorescence of the rhodamine B moiety as well as the coumarin was simultaneously quenched by Cu2+ due to the heavy metal ion effect [41]. In MeOH, upon addition of 20 eq. Cr3+ and Fe3+, an obvious fluorescence increment at 579 nm and a moderate fluorescence reduction at 470 nm was observed, indicating the generation of a FRET process between coumarin and rhodamine B. The emission intensity change ratios induced by Fe3+ and Cr3+ were 59-fold and 22-fold, respectively. In MeCN, only Cr3+ could induce the fluorescence increment of Rh-C at 581 nm, suggesting that Rh-C could selectively detect Cr3+ in MeCN via fluorimetry. In THF, the fluorescence of Rh-C showed almost no changes upon addition of the tested metal ions. In short, Rh-C exhibited a moderate selectivity toward Fe3+ in MeOH and an outstanding selectivity toward Cr3+ in MeCN through a FRET pathway.
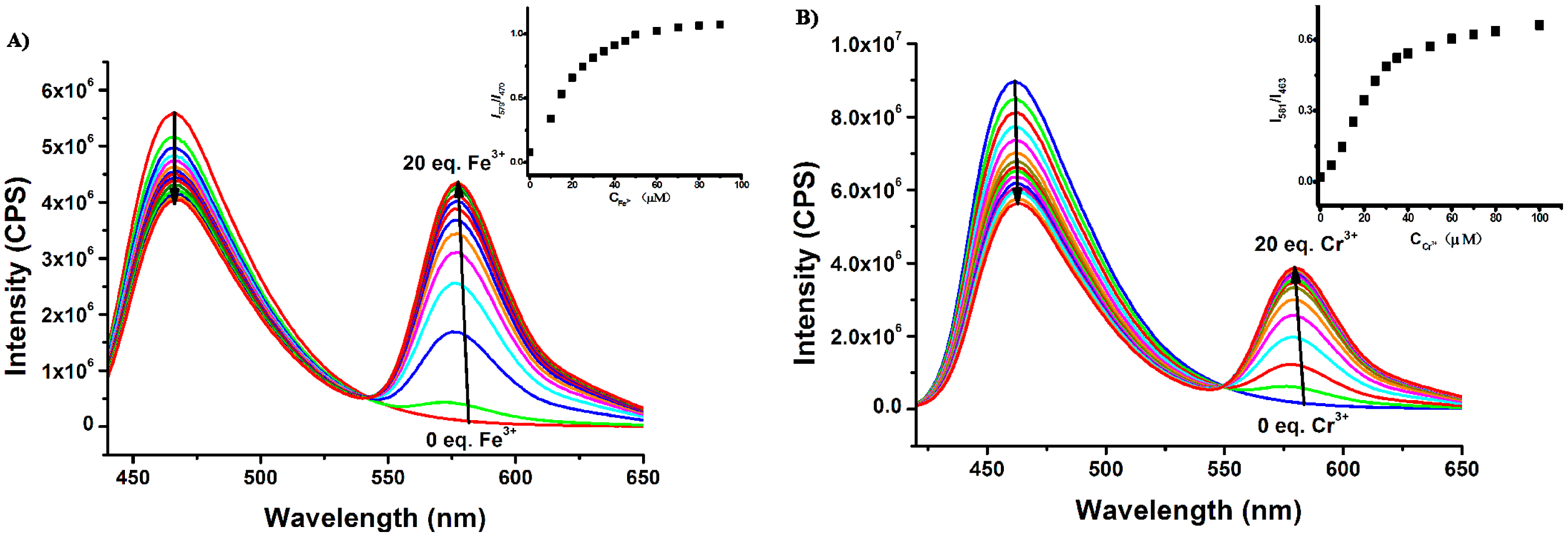
Subsequently, the fluorescence titrations of Rh-C (5 μM) toward Fe3+ in MeOH and Cr3+ in MeCN were exploited, respectively. As depicted in Figure 4, with the addition of more of Fe3+ into the MeOH solution of Rh-C, the emission of Rh-C at 470 nm gradually decreased, and a new peak at 579 nm corresponding to the spiro ring opening of rhodamine B appeared (Figure 4A). A FRET process was speculated to emerge between the coumarin moiety and the rhodamine B fluorophore. The fluorescence intensity ratio (I579/I470) reached a plateau when 10 equiv Fe3+ was added, accompanied by a remarkable enhancement of the emission intensity ratio from 0.02 to 0.94. A similar phenomenon was observed upon addition of Cr3+ into the MeCN solution of Rh-C. The emission of Rh-C at 463 nm decreased gradually as the amount of Cr3+ in MeCN increased, accompanied with a fluorescence increase at 581 nm (Figure 4B). The fluorescence intensity ratio (I581/I463) became stable after 10 equiv of Cr3+ was added, with an enhancement of the emission intensity ratio from 0.02 to 0.68.
We believe that the different selectivity of Rh-C towards metals in different solvents (THF, MeOH, and MeCN) is closely related to the complexing abilities of the metals. All the ions (Cu2+, Fe3+, and Cr3+) complexed with Rh-C could induce ring-opening of the rhodamine moiety. In addition, the coumarin moiety, which shows an absorption band that peaks at∼415 nm, behaves as an energy donor for the FRET system, and the open form of the rhodamine moiety, which shows an absorption band that peaks at ∼550 nm, acts as an energy acceptor.
3. Experimental Section
3.1. General Information
1H-NMR and 13C-NMR spectra were measured on a Bruker AM400 NMR spectrometer (Fällanden, Switzerland). Proton chemical shifts of NMR spectra are given in ppm relative to the internal reference TMS (1H, 0.00 ppm). ESI-MS and HRMS spectral data were recorded on a Thermo Finnigan LCQDECA (San Jose, CA, USA) and a BrukerDaltonics Bio TOF mass spectrometer (Billerica, MA, USA), respectively. Fluorescence emission spectra were obtained using FluoroMax-4 Spectrofluorophotometer (HORIBA JobinYvon, Paris, France) at 298 K. Unless otherwise noted, materials were obtained from commercial suppliers and were used without further purification. All of the solvents were either HPLC or spectroscopic grade in the optical spectroscopic studies and they were dried according to the standard methods prior to use.
3.2. Synthesis of Various Compounds
Rh-1, Rh-2, C-1 and C-2 were synthesized according to the literature [42–44].
Synthesis of 2-Azido-N-(3′,6′-bis(diethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)acetamide (Rh-3). Under nitrogen, Rh-2 (650 mg, 1.2 mmol) was dissolved in DMF (15 mL), then sodium azide (234 mg, 3.6 mmol) was added to the solution very carefully. The mixture was stirred at 50 °C for 12 h. After the reaction, the solution extracted with CH2Cl2 (3 × 20 mL) and the combined organic layer was washed with brine (3 × 20 mL). After the solution was dried over anhydrous sodium sulfate, the solvent was removed under the reduced pressure and the residue was further purified by column chromatography to afford Rh-3 (620 mg, 95.6%) as a white solid. 1H-NMR (400 MHz, DMSO) δ 9.91 (s, 1H), 7.84 (d, J = 6.9 Hz, 1H), 7.63–7.48 (m, 2H), 7.03 (d, J = 7.2 Hz, 1H), 6.51 (d, J = 9.3 Hz, 2H), 6.34 (d, J = 6.6 Hz, 4H), 3.99 (s, 2H), 3.34 (s, 8H), 1.09 (t, J = 6.8 Hz, 13H). MS: m/z [M+H]+ calcd. 540.2719 found, 540.2723.
Synthesis of 7-(Diethylamino)-2-oxo-N-(prop-2-yn-1-yl)-2H-chromene-3-carboxamide (C-3). Under nitrogen, triethylamine (1.4 mL, 10 mmol) was added to a solution of propargylamine (0.68 mL, 10 mmol) in anhydrous DCM and then a solution of C-2(1.4 g, 5 mmol) in anhydrous DCM was added dropwise to the solution with stirring. The organic layer was washed with water after stirred for 24 h. After the solution was dried over anhydrous sodium sulfate, the solvent was removed under the reduced pressure and the residue was further purified by column chromatography to afford C-3 (1.3 g, 87.2%) as a yellow solid. 1H-NMR (400 MHz, CDCl3) δ 9.01 (s, 1H), 8.72 (s, 1H), 7.45 (d, J = 9.0 Hz, 1H), 6.67 (dd, J = 9.0, 2.5 Hz, 1H), 6.52 (d, J = 2.4 Hz, 1H), 4.25 (dd, J = 5.4, 2.5 Hz, 2H), 3.48 (q, J = 7.1 Hz, 4H), 2.26 (t, J = 2.5 Hz, 1H), 1.26 (t, J = 7.1 Hz, 6H).
Synthesis of N-((1-(2-((3′,6′-bis(diethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)amino)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-7-(diethylamino)-2-oxo-2H-chromene-3-carboxamide (Rh-C). Rh-3 (369 mg, 0.68 mmol), C-3 (210 mg, 0.7 mmol) and CuI (285 mg, 1.5 mmol) were added in 20 mL THF-water (v:v, 1:1). The mixture was stirred at 50 °C for 24 h, and monitored by TLC. Then the solution was cooled down to room temperature and the solvent was removed under reduced pressure. After that, the solution was poured into distilled water and extracted with CH2Cl2 (3 × 30 mL). The combined extracts were dried over anhydrous sodium sulfate. The solvent was removed under the reduced pressure and the residue was further purified by column chromatography to afford Rh-C (204 mg, 35.8%) as an orange solid. 1H-NMR (400 MHz, DMSO) δ 10.12 (s, 1H), 9.02 (t, J = 5.5 Hz, 1H), 8.70 (s, 1H), 7.82 (d, J = 6.8 Hz, 1H), 7.73–7.67 (m, 2H), 7.61–7.48 (m, 2H), 7.01 (d, J = 7.3 Hz, 1H), 6.82 (dd, J = 9.1, 2.3 Hz, 1H), 6.63 (d, J = 2.0 Hz, 1H), 6.52 (d, J = 8.5 Hz, 2H), 6.34 (d, J = 9.2 Hz, 4H), 5.07 (s, 2H), 4.54 (d, J = 5.5 Hz, 2H), 3.49 (q, J = 6.9 Hz, 4H), 3.32 (d, J = 7.7 Hz, 8H), 1.15 (t, J = 7.0 Hz, 6H), 1.08 (t, J = 6.9 Hz, 12H). MS: m/z [M+H]+, calcd. 838.4041, found 838.4021.
4. Conclusions
In conclusion, we have developed a fluorescent chemosensor Rh-C based on a rhodamine B and coumarin backbone. This probe allowed the selective detection Cu2+ in different solvents (THF, MeOH and MeCN) by the naked eye, especially in THF. Additionally, Rh-C could also sense Fe3+ in MeOH and Cr3+ in MeCN by ratiometric fluorescence change via a FRET process. Hence, we realize the optical detection of three kinds of common heavy metal ions with a single probe molecule, which might prove beneficial for the strategic design of multifunctional optical sensors.
Supplementary Materials
Supplementary materials can be accessed at: https://www.mdpi.com/1424-8220/15/1/49/s1.
Acknowledgments
This work was financially supported by the National Science Foundation of China (91026022) and China Postdoctoral Science Foundation funded project (2014M560718).
Author Contributions
In this paper, KL, KY and SL designed research; YY, LY and JL performed research and analyzed the data; KY and KL wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Reference
- Wu, X.; Guo, Z.; Wu, Y.; Zhu, S.; James, T.D.; Zhu, W. Near-infrared colorimetric fluorescent Cu2+ sensors based on indoline–benzothiadiazole derivatives via formation of radical cations. ACS Appl. Mater. Interfaces 2013, 5, 12215–12220. [Google Scholar]
- Kagit, R.; Yildirim, M.; Ozay, O.; Yesilot, S.; Ozay, H. Phosphazene based multicentered naked-eye fluorescent sensor with high selectivity for Fe3+ ions. Inorg. Chem. 2014, 53, 2144–2151. [Google Scholar]
- Li, J.; Han, C.; Wu, W.; Zhang, S.; Guo, J.; Zhou, H. Selective and cyclic detection of Cr3+ using poly(methylacrylic acid) monolayer protected gold nanoparticles. New J. Chem. 2014, 38, 717–722. [Google Scholar]
- Liu, Z.; Zhang, C.; Wang, X.; He, W.; Guo, Z. Design and synthesis of a ratiometric fluorescent chemosensor for Cu(II) with a fluorophore hybridization approach. Org. Lett. 2012, 14, 4378–4381. [Google Scholar]
- Fan, J.; Zhan, P.; Hu, M.; Sun, W.; Tang, J.; Wang, J.; Sun, S.; Song, F.; Peng, X. A fluorescent ratiometric chemodosimeter for Cu2+ based on TBET and its application in living Cells. Org. Lett. 2013, 15, 492–495. [Google Scholar]
- Jo, J.; Lee, H.Y.; Liu, W.; Olasz, A.; Chen, C.H.; Lee, D. Reactivity-based detection of Copper(II) ion in water: Oxidative cyclization of azoaromatics as fluorescence turn-on signaling mechanism. J. Am. Chem. Soc. 2012, 134, 16000–16007. [Google Scholar]
- Bricks, J.L.; Kovalchuk, A.; Trieflinger, C.; Nofz, M.; Büschel, M.; Tolmachev, A.I.; Daub, J.; Rurack, K. On the development of sensor molecules that display FeIII-amplified fluorescence. J. Am. Chem. Soc. 2005, 127, 13522–13529. [Google Scholar]
- En, D.; Guo, Y.; Chen, B.; Dong, B.; Peng, M.J. Coumarin-derived Fe3+-selective fluorescent turn-off chemosensors: synthesis, properties, and applications in living cells. RSC Adv. 2014, 4, 248–253. [Google Scholar]
- Huang, L.; Hou, F.; Cheng, J.; Xi, P.; Chen, F.; Bai, D.; Zeng, Z. Selective off–on fluorescent chemosensor for detection of Fe3+ ions in aqueous media. Org. Biomol. Chem. 2012, 10, 9634–9638. [Google Scholar]
- Kim, H.; Kim, K.B.; Song, E.J.; Hwang, I.H.; Noh, J.Y.; Kim, P.-G.; Jeong, K.-D.; Kim, C. Turn-on selective fluorescent probe for trivalent cations. Inorg. Chem. Commun. 2013, 36, 72–76. [Google Scholar]
- Wang, J.H.; Zhang, D.; Liu, Y.Q.; Ding, P.G.; Wang, C.C.; Ye, Y.; Zhao, Y.F. A N-stablization rhodamine-based fluorescent chemosensor for Fe3+ in aqueous solution and its application in bioimaging. Sens. Actuator B Chem. 2014, 191, 344–350. [Google Scholar]
- Xie, P.; Guo, F.; Xia, R.; Wang, Y.; Yao, D.; Yang, G.; Xie, L. A rhodamine-dansyl conjugate as a FRET based sensor for Fe3+ in the red spectral region. J. Lumin. 2014, 145, 849–854. [Google Scholar]
- Liu, D.; Pang, T.; Ma, K.; Jiang, W.; Bao, X. A new highly sensitive and selective fluorescence chemosensor for Cr3+ based on rhodamine B and a 4,13-diaza-18-crown 6-ether conjugate. RSC Adv. 2014, 4, 2563–2567. [Google Scholar]
- Kaur, M.; Kaur, P.; Dhuna, V.; Singhb, S.; Singh, K. A ferrocene–pyrene based “turn-on” chemodosimeter for Cr3+—application in bioimaging. Dalton Trans. 2014, 43, 5707–5712. [Google Scholar]
- Zhou, Z.; Yu, M.; Yang, H.; Huang, K.; Li, F.; Yi, T.; Hong, C. FRET-based sensor for imaging chromium(III) in living cells. Chem. Commun. 2008, 3387–3389. [Google Scholar]
- Hargrove, A.E.; Nieto, S.; Zhang, T.; Sessler, J.L.; Anslyn, E.V. Artificial receptors for the recognition of phosphorylated molecules. Chem. Rev. 2011, 111, 6603–6782. [Google Scholar]
- Quang, D.T.; Kim, J.S. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar]
- Zhang, J.F.; Zhou, Y.; Yoon, J.; Kim, J.S. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 2011, 40, 3416–3429. [Google Scholar]
- Du, J.; Hu, M.; Fan, J.; Peng, X. Fluorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012, 41, 4511–4535. [Google Scholar]
- Chereddy, N.R.; Janakipriya, S.; Korrapati, P.S.; Thennarasu, S.; Mandal, A.B. Solvent-assisted selective detection of sub-micromolar levels of Cu2+ ions in aqueous samples and live-cells. Analyst 2013, 138, 1130–1136. [Google Scholar]
- Niamnont, N.; Kimpitak, N.; Tumcharern, G.; Rashatasakhon, P.; Sukwattanasinitt, M. Highly sensitive salicylic fluorophore for visual detection of picomole amounts of Cu2+. RSC Adv. 2013, 3, 25215–25220. [Google Scholar]
- Chen, S.; Hou, P.; Foley, J.W.; Song, X. A colorimetric and ratiometric fluorescent probe for Cu2+ with a large red shift and its imaging in living cells. RSC Adv. 2013, 3, 5591–5596. [Google Scholar]
- Yu, F.; Zhang, W.; Li, P.; Xing, Y.; Tong, L.; Ma, J.; Tang, B. Cu2+-selective naked-eye and fluorescent probe: its crystal structure and application in bioimaging. Analyst 2009, 134, 1826–1833. [Google Scholar]
- Sahoo, S.K.; Sharma, D.; Bera, R.K.; Crisponi, G.; Callan, J.F. Iron(III) selective molecular and supramolecular fluorescent probes. Chem. Soc. Rev. 2012, 41, 7195–7227. [Google Scholar]
- Ye, J.H.; Wang, Y.; Bai, Y.; Zhang, W.; He, W. A highly selective turn-on fluorescent chemodosimeter for Cr(VI) and its application in living cell imaging. RSC Adv. 2014, 4, 2989–2992. [Google Scholar]
- Xu, Y.; Yang, W.; Shao, J.; Zhou, W.; Zhu, W.; Xie, J. A simple donor-acceptor probe for the detection of Cr3+ cations. RSC Adv. 2014, 4, 15400–15405. [Google Scholar]
- Guha, S.; Lohar, S.; Banerjee, A.; Sahana, A.; Mukhopadhyay, S.K.; Matalobos, J.S.; Das, D. Anthracene appended coumarin derivative as a Cr(III) selective turn-on fluorescent probe for living cell imaging: a green approach towards speciation studies. Anal. Methods 2012, 4, 3163–3168. [Google Scholar]
- Xie, P.; Guo, F.; Xiao, Y.; Jin, Q.; Yao, D.; Huang, Z. A fluorescent chemosensor based on rhodamine for Cr3+ in red spectral region in aqueous solutions and living cells. J. Lumin. 2013, 140, 45–50. [Google Scholar]
- Wan, X.; Liu, H.; Yao, S.; Liu, T.; Yao, Y. A stimuli-responsive nanogel-based sensitive and selective fluorescent sensor for Cr3+ with thermo-induced tunable detection sensitivity. Macromol. Rapid Commun. 2014, 35, 323–329. [Google Scholar]
- Li, M.; Zhang, D.; Liu, Y.; Ding, P.; Ye, Y.; Zhao, Y. A. Novel Colorimetric and Off–On Fluorescent Chemosensor for Cr3+ in Aqueous Solution and its Application in Live Cell Imaging. J. Fluoresc. 2014, 24, 119–127. [Google Scholar]
- Kim, H.N.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465–1472. [Google Scholar]
- Beija, M.; Afonso, C.A.M.; Martinho, J.M.G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar]
- Hou, J.T.; Li, K.; Liu, B.Y.; Liao, Y.X.; Yu, X.Q. The first ratiometric probe for lysine in water. Tetrahedron 2013, 69, 2118–2123. [Google Scholar]
- Yuan, L.; Lin, W.; Xie, Y.; Chen, B.; Song, J. Development of a ratiometric fluorescent sensor for ratiometric imaging of endogenously produced nitric oxide in macrophage cells. Chem. Commun. 2011, 47, 9372–9374. [Google Scholar]
- Lohar, S.; Banerjee, A.; Sahana, A.; Banik, A.; Mukhopadhyay, S.K.; Das, D. A rhodamine–naphthalene conjugate as a FRET based sensor for Cr3+ and Fe3+ with cell staining application. Anal. Methods 2013, 5, 442–445. [Google Scholar]
- Guan, X.; Lin, W.; Huang, W. Development of a new rhodamine-based FRET platform and its application as a Cu2+ probe. Org. Biomol. Chem. 2014, 12, 3944–3949. [Google Scholar]
- Hou, J.T.; Zhang, Q.F.; Xu, B.Y.; Lu, Q.S.; Liu, Q.; Zhang, J.; Yu, X.Q. A novel BINOL-based cyclophane via click chemistry: synthesis and its applications for sensing silver ions. Tetrahedron Lett. 2011, 52, 4927–4930. [Google Scholar]
- Kim, H.; Lee, S.; Lee, J.; Tae, J. Rhodamine triazole-based fluorescent probe for the detection of Pt2+. Org. Lett. 2010, 12, 5342–5345. [Google Scholar]
- Chang, K.C.; Su, I.H.; Senthivelan, A.; Chung, W.S. Triazole-modified calix[4]crown as a novel fluorescent on–off switchable chemosensor. Org. Lett. 2007, 9, 3363–3366. [Google Scholar]
- Upadhyay, K.K.; Kumar, A. Pyrimidine based highly sensitive fluorescent receptor for Al3+ showing dual signalling mechanism. Org. Biomol. Chem. 2010, 8, 4892–4897. [Google Scholar]
- Liu, Y.L.; Lv, X.; Zhao, Y.; Liu, J.; Sun, Y.Q.; Wang, P.; Guo, W. A Cu (II)-based chemosensing ensemble bearing rhodamine B fluorophore for fluorescence turn-on detection of cyanide. J. Mater. Chem. 2012, 22, 1747–1750. [Google Scholar]
- Hou, J.T.; Li, K.; Yu, K.K.; Ao, M.Z.; Wang, X.; Yu, X.Q. Novel triazole-based fluorescent probes for Pd2+ in aqueous solutions: design, theoretical calculations and imaging. Analyst 2013, 138, 6632–6638. [Google Scholar]
- Ji, S.; Meng, X.; Ye, W.; Feng, Y.; Sheng, H.; Cai, Y.; Liu, J.; Zhu, X.; Guo, Q. A rhodamine-based “turn-on” fluorescent probe for Fe3+ in aqueous solution. Dalton Trans. 2014, 43, 1583–1588. [Google Scholar]
- He, G.J.; Guo, D.; He, C.; Zhang, X.L.; Zhao, X.W.; Duan, C.Y. A color-tunable Europium complex emitting three primary colors and white light. Angew. Chem. Int. Ed. 2009, 48, 6132–6135. [Google Scholar]

© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yu, K.; Yang, L.; Liu, J.; Li, K.; Luo, S. One Single Molecule as a Multifunctional Fluorescent Probe for Ratiometric Sensing of Fe3+, Cr3+ and Colorimetric Sensing of Cu2+. Sensors 2015, 15, 49-58. https://doi.org/10.3390/s150100049
Yang Y, Yu K, Yang L, Liu J, Li K, Luo S. One Single Molecule as a Multifunctional Fluorescent Probe for Ratiometric Sensing of Fe3+, Cr3+ and Colorimetric Sensing of Cu2+. Sensors. 2015; 15(1):49-58. https://doi.org/10.3390/s150100049
Chicago/Turabian StyleYang, Yanqiu, Kangkang Yu, Liang Yang, Jun Liu, Kun Li, and Shunzhong Luo. 2015. "One Single Molecule as a Multifunctional Fluorescent Probe for Ratiometric Sensing of Fe3+, Cr3+ and Colorimetric Sensing of Cu2+" Sensors 15, no. 1: 49-58. https://doi.org/10.3390/s150100049



