Detection of Quorum Sensing Activity in the Multidrug-Resistant Clinical Isolate Pseudomonas aeruginosa Strain GB11
Abstract
: A multidrug-resistant clinical bacteria strain GB11 was isolated from a wound swab on the leg of a patient. Identity of stain GB11 as Pseudomonas aeruginosa was validated by using matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS). Detection of the production of signaling molecules, N-acylhomoserine lactones (AHLs), was conducted using three different bacterial biosensors. A total of four different AHLs were found to be produced by strain GB11, namely N-butyryl homoserine lactone (C4-HSL), N-hexanoylhomoserine lactone (C6-HSL), N-octanoyl homoserine lactone (C8-HSL) and N-3-oxo-dodecanoylhomoserine lactone (3-oxo-C12-HSL) using high resolution liquid chromatography tandem mass spectrometry (LC-MS/MS). Of these detected AHLs, 3-oxo-C12-HSL was found to be the most abundant AHL produced by P. aeruginosa GB11.1. Introduction
Bacterial cell-to-cell communication (quorum sensing, hereafter QS) modulates the regulation of the network of physiological activities in relation to the population density by synchronizing the concentration of signaling molecules (also known as “autoinducers”) [1,2]. QS is employed by bacteria to sense and response to any changes in the environment through secretion of signaling molecules. When a critical threshold of signaling molecules concentration is achieved, various targeted gene will be regulated either by activating or inhibiting a cascade of gene expression [3]. There are different signaling molecules employed by Gram-negative and Gram-positive bacteria which are N-acylhomoserine lactones (AHLs) and oligopeptide molecules, respectively. The QS mechanism utilized by Gram-negative bacteria typically involves a LuxI family protein as “autoinducer” synthase and LuxR family protein as transcriptional activator [4]. QS had been reported in many different types of bacteria to regulate bacterial physiological activities including those phenotypic adaptations to the environment [5–8].
Most Gram-negative pathogenic bacteria possess the ability of producing AHLs that allow them to control virulence genes that could affect bacterial-host interactions [8]. The ability of eukaryotic hosts to produce signals that mimic AHLs suggests that the hosts have adapted and are able to detect the extracellular AHLs [8]. This suggests that the AHL could be the “interkingdom signals” that could make direct interactions between host and bacteria. While Pseudomonas aeruginosa is a clinically significant and opportunistic pathogen which often associated with nosocomial infections in compromised hosts, it could be an attractive target for sorting out its communication system and developing QS inhibitors that could act as potential therapeutics [9–12]. P. aeruginosa acts as an important pathogen that can express its pathogenicity in almost any part of the human body and can be divided into two general categories which are loss of cell integrity and immune modulation [8]. The creation of biofilms, a bacterial activity regulated by QS with the production of signaling molecules, often renders Pseudomonas infections difficult to eradicate and more resistant towards antibiotics [13]. It is known that P. aeruginosa adopts a hierarchical network of QS systems to regulate biofilm formation and expression of virulence factors that involves the RhlI/R, LasI/R and PQS signalling systems [14–16]. Thus, investigating the QS molecules in P. aeruginosa is an important first step to further understanding this multidrug resistant pathogen and could be one of the means for generating cures for diseases caused by this opportunistic pathogen.
2. Experimental Section
2.1. Clinical Isolate
The bacterial strain GB11 was isolated from a wound swab taken from an infected leg ulcer of an 83 years old man. Gram stain initially showed scanty leucocytes with numerous gram negative rods and scanty gram positive cocci. Heavy growth of P. aeruginosa (initially identified using API® 20 NE) was obtained from the Blood Agar and McConkey Agar plates after overnight incubation. Antimicrobial Susceptibility Testing following the Clinical and Laboratory Standards Institute (CSLI) method was performed [17]. The isolate was sensitive to amikacin, gentamicin, netilmicin, tobramycin and imipenem, but resistant to ceftazidime, cefoperazone and ciprofloxacin.
2.2. Bacterial Strains and Culture Conditions
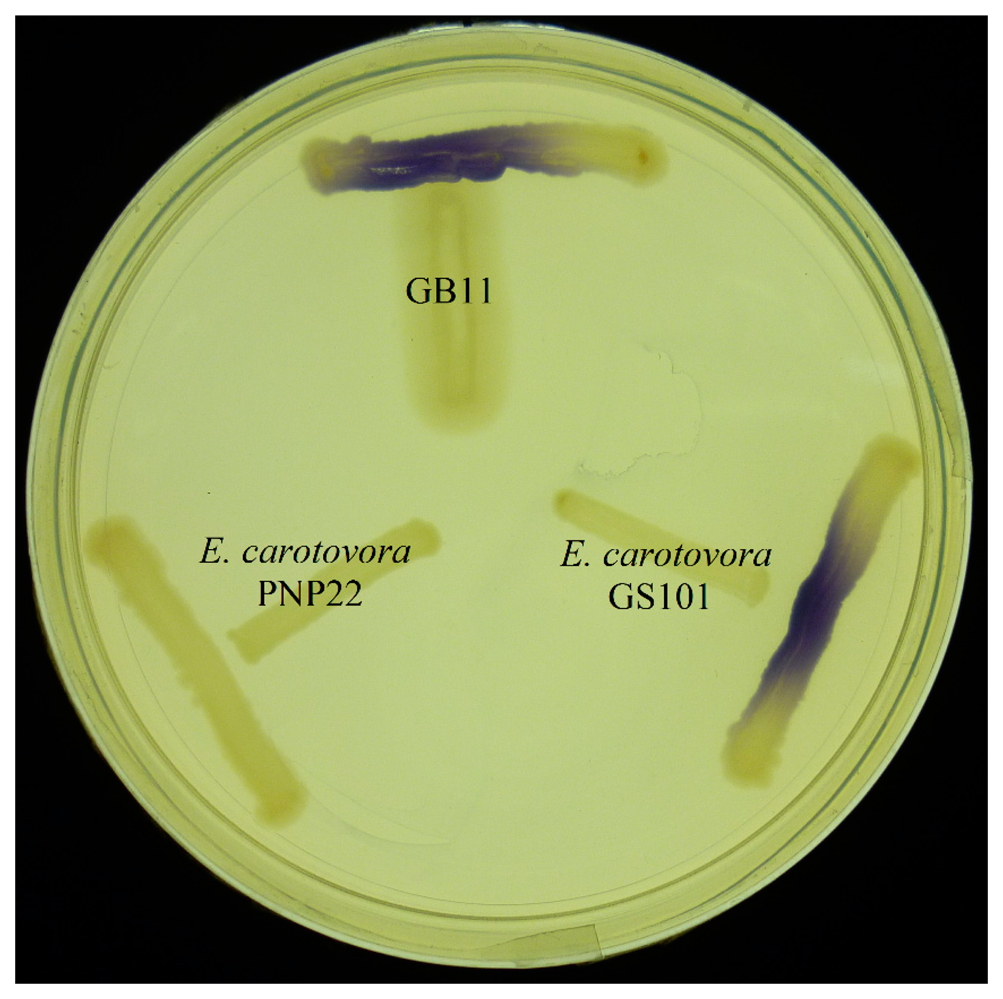
Bacteria strain GB11 was cultured aerobically at 37 °C in Tryptic soy medium (TSm) which consists of casein peptone 15.0 (mg/mL), soy peptone 5.0 (mg/mL), sodium chloride 5.0 (mg/mL), and agar 15.0 (mg/mL). All other strains, including three biosensors and two controls, were cultured aerobically in LB agar and LB medium (LBm) at 28 °C as described previously [18–20], unless stated otherwise. The three biosensors are Chromobacterium violaceum CV026 [21], Escherichia coli [pSB401] and E. coli [pSB1075] [22], which were used for the detection of AHLs while Erwinia carotovora GS101 and E. carotovora PNP22 act as positive and negative controls, respectively.
2.3. Preparation and Screening of AHL by C. violaceum CV026 Biosensor
All AHLs used were obtained as described previously [23–25]. Briefly, AHL stock solutions were prepared with acetonitrile (ACN) (Merck, Frankfurt, Germany) to a concentration of 1 g/L. AHL solutions were stored at −20 °C for not more than a month. The stock solutions were then diluted with ACN to 100 ppm as standard in liquid chromatography mass spectrometry analysis. Production of AHL obtained from strain GB11 was screened using cross-streaking with the biosensor C. violaceum CV026 on LBm agar.
2.4. AHL Extraction
Bacteria strain GB11 was cultured overnight for 18 h in LBm as illustrated previously [23]. Briefly, 50 mM of 3-[N-morpholino] propanesulfonic acid (MOPS) was used to buffer the culture media to pH 5.5 at 37 °C. Extraction of supernatant was conducted twice with equal volume of ethyl acetate buffered with 0.1% v/v glacial acetic acid. AHL extracts were dried completely before further analysis.
2.5. Bioluminescence Assay
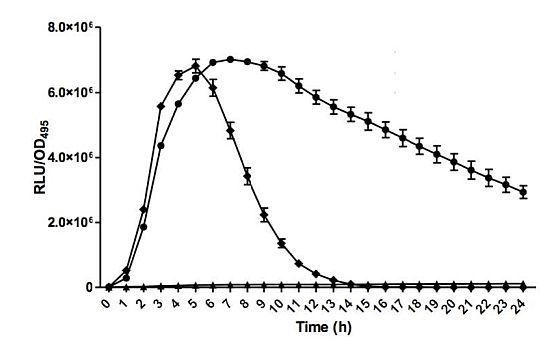
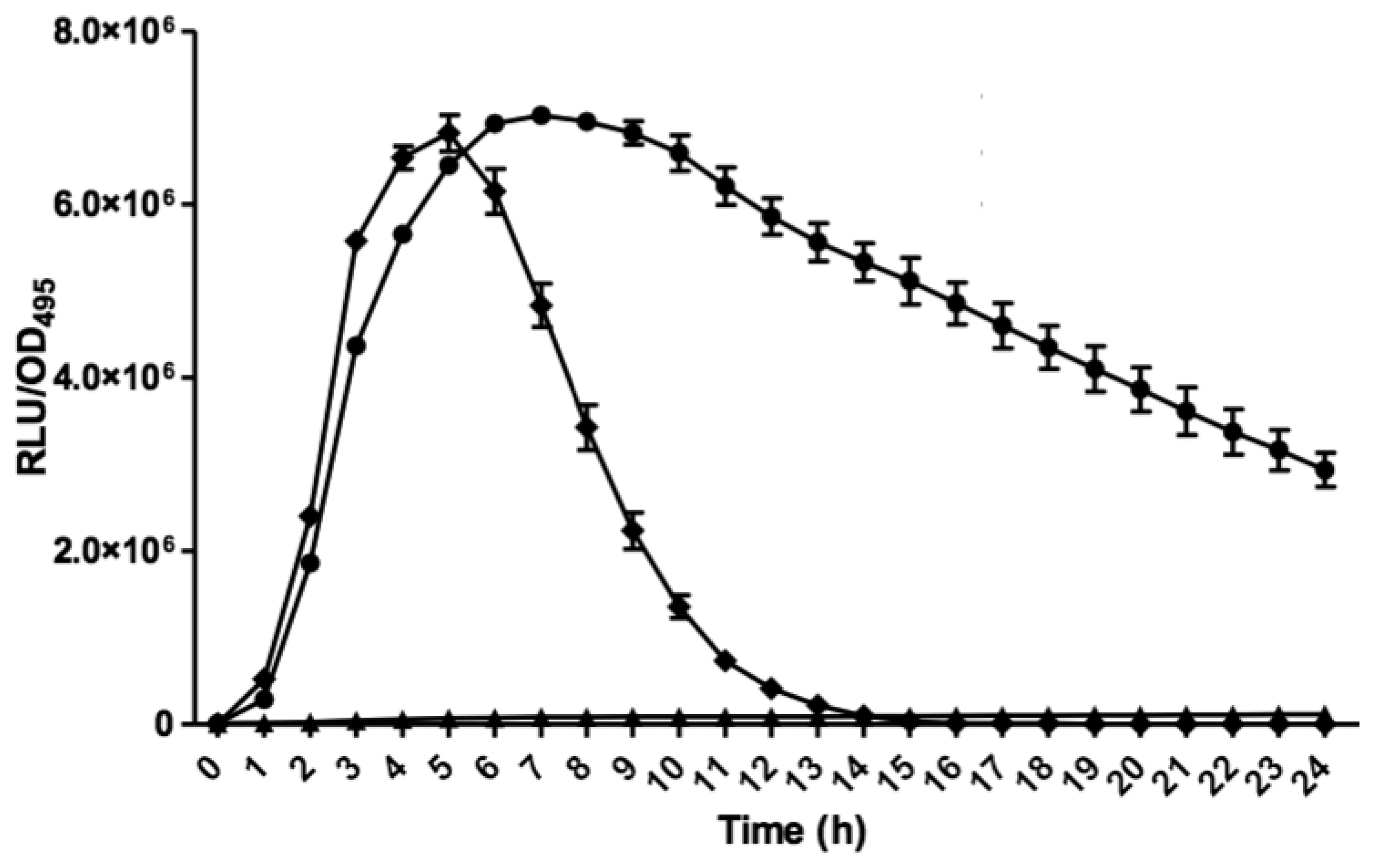
Bioluminescence assay was conducted with Tecan luminometer (Infinite M200, Männerdorf, Switzerland) as described previously [26,27]. Briefly, 250 μL of diluted E. coli biosensor (OD600 = 0.1) was used to resuspend the AHL extract. Bioluminescence and optical density (OD495) readings were documented simultaneously every 60 min interval for 24 h and experiments were repeated three times with triplicates for each repeat. Bioluminescence measurement was calculated as relative light unit per OD495 (RLU/OD495) against 24 h [22].
2.6. MALDI-TOF MS for Strain Identification
Fresh culture of bacteria strain GB11 was identified by MALDI-TOF (Bruker Daltonik GmbH, Leipzig, Germany) using Bruker FlexControl software version 3.3 (Build 108) as described previously [28].
2.7. High Resolution Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) Analysis
High Resolution Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) was conducted as reported previously [20,24,25]. Briefly, an Agilent 1290 Infinity LC system (Agilent Technologies Inc., Santa Clara, CA, USA) was equipped with a C18 column. Then, 2 μL of sample was injected into the system for analysis. Water and ACN were used as the solvents for mobile phases. HPLC gradient profiles were set as: 0 min: 4:1, 7 min: 1:1, 12 min: 1:4, and 14 min: 4:1. For AHL detection, precursor ion scan mode was used and the product ion m/z ratio was set as 102 indicating the [M+H]+ ion of the core lactone ring where the precursor ions were then scanned from 150 to 400. Thus, various AHLs were identified based on the detection of the fragmentation of the core HSL moiety in the collision cell.
3. Results and Discussion
3.1. Detection of AHL Production in Strain GB11
The ability of a variety of bacterial biosensors has paved a pathway for researching and understanding bacterial QS properties and be able to provide a fast and accurate way for detecting the production of AHLs. The AHL biosensor practically relies on the LuxR protein and displays a specific attraction towards the cognate AHL and positively regulates the transcriptional of targeted gene [29]. CV026 biosensor is a white colony C. violaceum mutant [21]. It is most sensitive to C6-HSL, its natural AHL produced by wild-type C. violaceum, however it can also detect short-chain AHLs ranging from 4 to 8 carbon side-chain AHLs with or without C-3 substitution. Although the CV026 bioassay is comparatively easy to perform, false-negative results occasionally happen when some sample isolates produce bactericides that might kill CV026 [22]. Hence, bioassays using CV026 should be coupled with other analytical methods such as mass spectrometry. Also, other types of AHL biosensors should be used in addition to CV026.
E. coli harbouring the pSB401 and pSB1075 plasmids are chosen as alternative AHL biosensors. Plasmid pSB401 is a pACYC184 plasmid containing a fusion of luxRluxl'∷luxCDABE while plasmid pSB1075 is a pUC18 plasmid containing lasRlasl'∷luxCDABE whereby E. coli [pSB401] and E. coli [pSB1075] are capable of detecting short-chain AHLs and long-chain AHLs, respectively [22]. Strain GB11 showed induction of purple violacein in CV026 biosensor after incubation for 24 h that indicated the presence of short-chain AHLs (Figure 1). Bioluminescene bioassays were conducted to validate this results with increase of bioluminescence activity in both E. coli [pSB401] and E. coli [pSB1075] (Figure 2).
3.2. Identity of Strain GB11
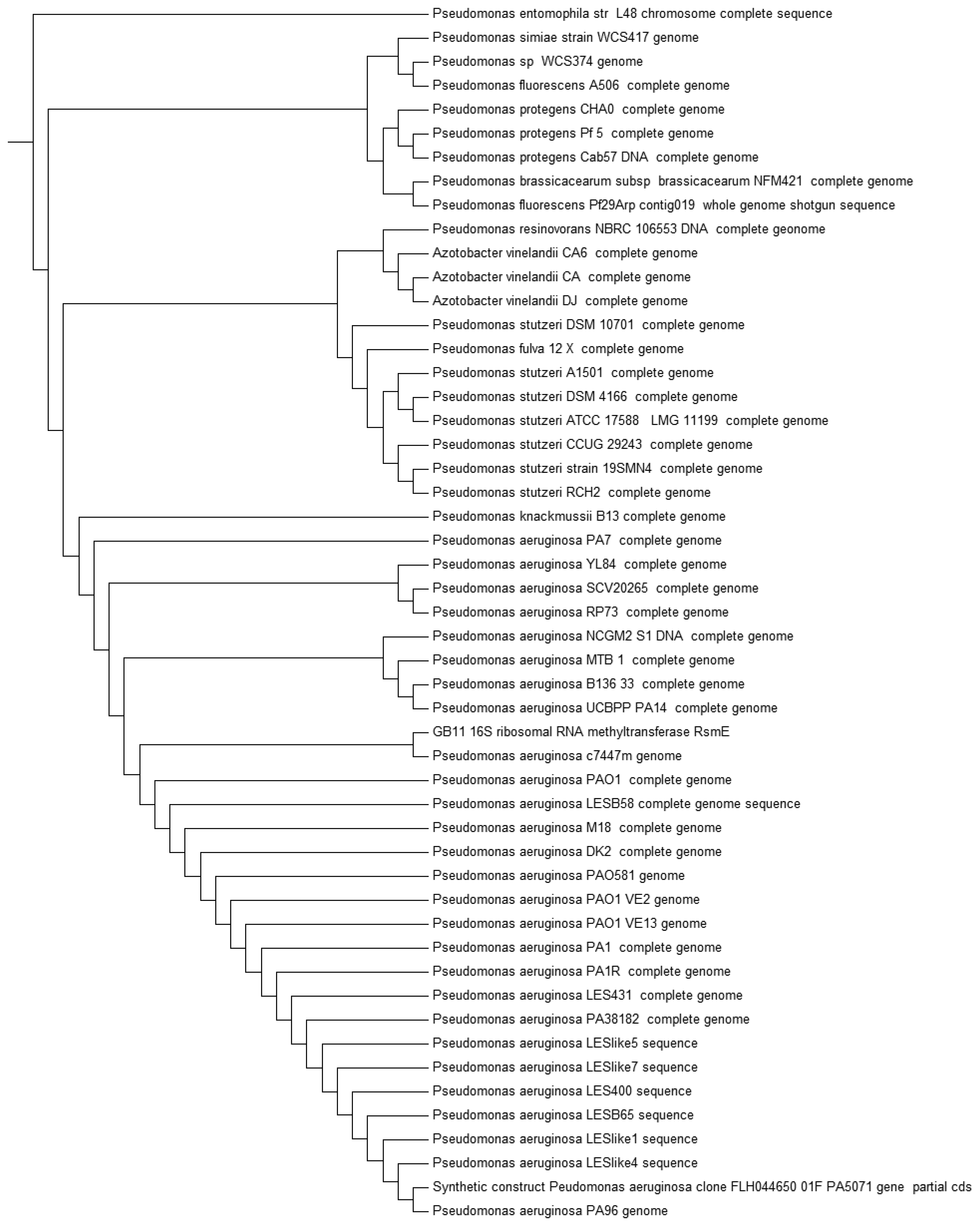
Recent advances ease the path on bacteria identification with established technique such as MALDI-TOF, which is one of the fastest mean to classify bacteria [30,31]. Strain GB11 was identified as Pseudomonas aeruginosa as the best match in MALDI-TOF identification (Figure 3). Our MALDI-TOF results is in agreement with the preliminary microbiological assessment of strain GB11. Later, we have verified the identity of strain GB11 using 16S rRNA gene analysis where a phylogeny tree was constructed by comparing the closely-related sequences from NCBI database (Figure 4).
3.3. AHL Profile Analysis by High Resolution Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
P. aeruginosa is an opportunistic pathogen that often infects patients with immunocompromised conditions such as those with cancers, burns or cystic-fibrosis [32]. QS is believed to be adopted by P. aeruginosa to invade and infect its host. The expression of many genes in P. aeruginosa are known to be regulated by QS such as lasA (onset of proteolysis and elastolysis) [33] and toxA [34]. Thus, inhibition of P. aeruginosa QS had been proposed as the promising alternative to attenuate its virulence and biofilm formation [35–37]. Understanding the types of AHLs produced by P. aeruginosa, is a milestone bringing us closer to finding effective ways to treat P. aeruginosa infections.
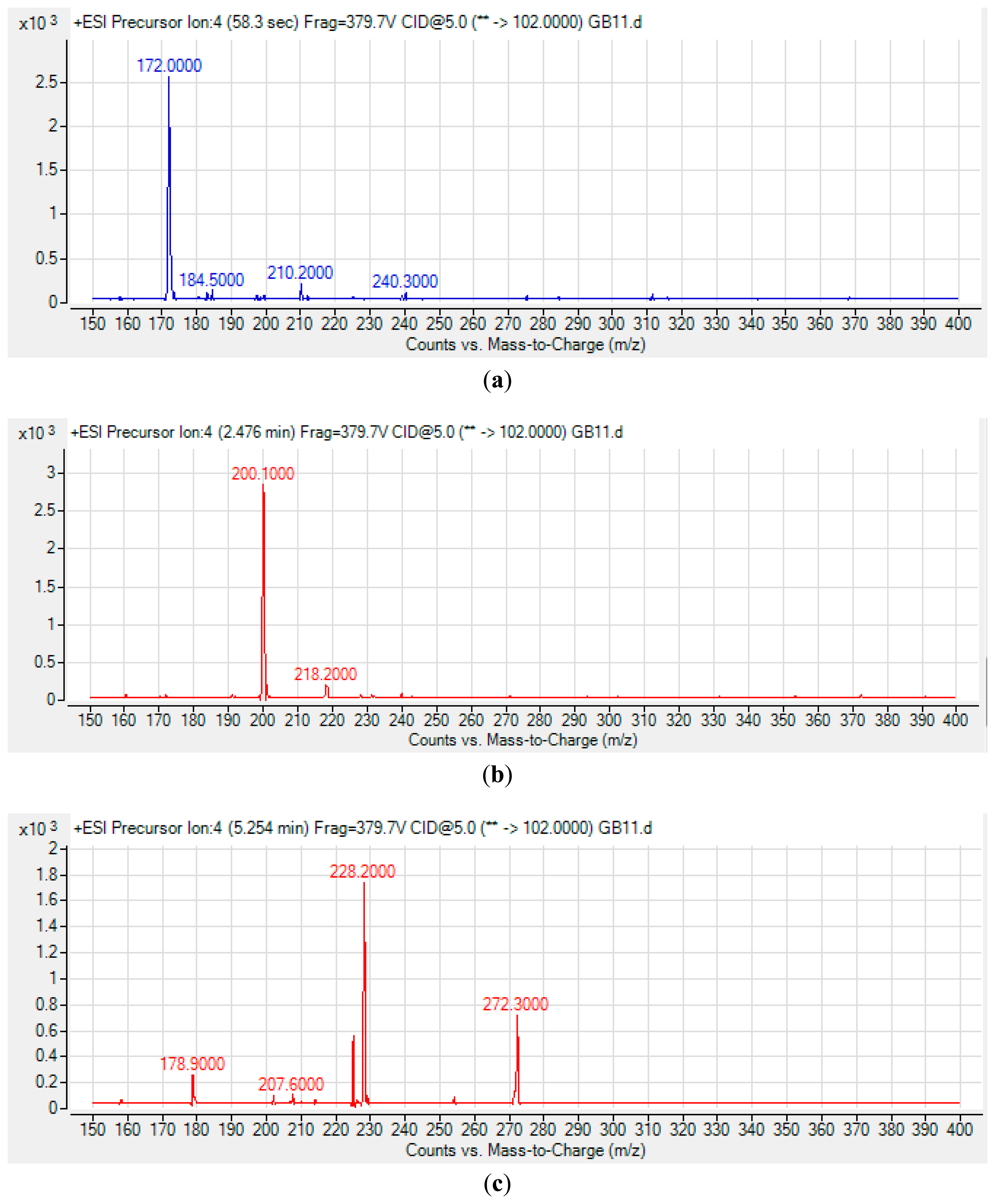
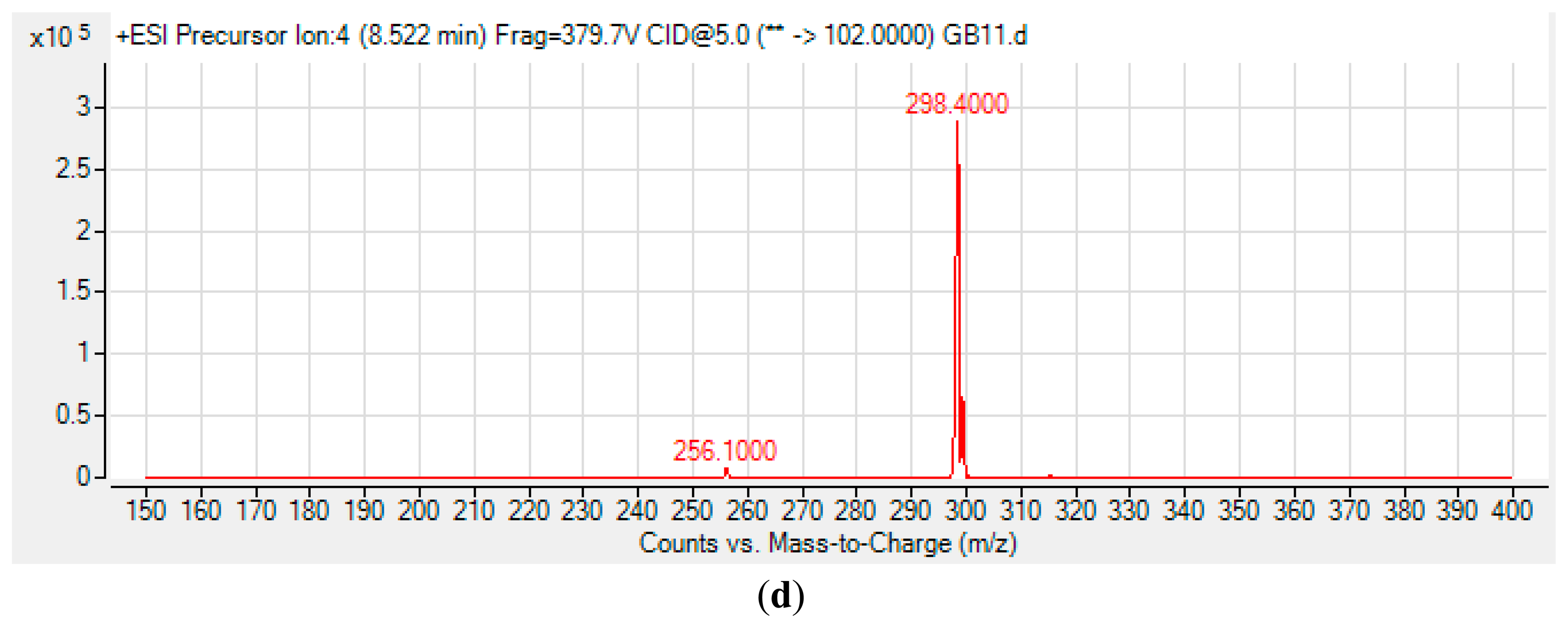
The spent culture of GB11 strain was analyzed using LC-MS/MS and this confirmed that P. aeruginosa GB11 produced four different types of AHL molecules which are N-butyryl-L-homoserine lactone (C4-HSL), N-hexanoyl-L-homoserine lactone (C6-HSL), N-octanoyl-L-homoserine lactone (C8-HSL) and N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL) (Figure 5). Of these detected AHLs, 3-oxo-C12-HSL is the most abundant AHL produced by P. aeruginosa GB11 and C8-HSL being the least produced AHL by this isolate. To our best knowledge, this is the first report on multiple production of these AHLs by P. aeruginosa. We believed that the investigation on AHL profile of P. aeruginosa GB11 would be important to understand the QS mechanism particularly virulent determinants regulation in this multidrug resistant clinical isolate.
4. Conclusions
QS plays a vital role in bacteria by regulating their physiological activities so that they are able to adapt to the environment and threaten their hosts. This work illustrated the importance in expanding the research on AHL-producing pathogens isolated from human wounds.
Acknowledgments
This work was supported by the University of Malaya and Kok-Gan Chan thanks the High Impact Research Grant (UM.C/625/1/HIR/MOHE/CHAN/01, Grant No. A-000001-50001) for the research funding provided.
Author Contributions
Yuet Meng Cheong collected the clinical bacterial strain and performed the initial work. Robson Ee and Huey Jia Cheng performed the experiments, Robson Ee, Wai-Fong Yin, Wen-Si Tan, Yuet Meng Cheong and Kok-Gan Chan prepared the draft. Kok-Gan Chan applied the funding and supervised the project. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atkinson, S.; Williams, P. Quorum sensing and social networking in the microbial world. J. Royal Soc. Interface 2009, 6, 959–978. [Google Scholar]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar]
- Wong, C.S.; Yin, W.F.; Choo, Y.M.; Sam, C.K.; Koh, C.L.; Chan, K.G. Coexistence of quorum-quenching and quorum-sensing in tropical marine Pseudomonas aeruginosa strain MW3A. World J. Microbiol. Biot. 2012, 28, 453–461. [Google Scholar]
- Swift, S.; Downie, J.A.; Whitehead, N.A.; Barnard, A.M.; Salmond, G.P.; Williams, P. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microbial. Physiol. 2001, 45, 199–270. [Google Scholar]
- Shrout, J.D.; Chopp, D.L.; Just, C.L.; Hentzer, M.; Givskov, M.; Parsek, M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006, 62, 1264–1277. [Google Scholar]
- Chan, K.G.; Puthucheary, S.D.; Chan, X.Y.; Yin, W.F.; Wong, C.S.; Too, W.S.; Chua, K.H. Quorum sensing in Aeromonas species isolated from patients in Malaysia. Curr. Microbiol. 2011, 62, 167–172. [Google Scholar]
- Zhang, L.; Murphy, P.J.; Kerr, A.; Tate, M.E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature 1993, 362, 446–448. [Google Scholar]
- Teplitski, M.; Mathesius, U.; Rumbaugh, K.P. Perception and degradation of N-acyl homoserine lactone quorum sensing signals by mammalian and plant cells. Chem. Rev. 2011, 111, 100–116. [Google Scholar]
- Gales, A.C.; Sader, H.H.; Jones, R.N. Respiratory tract pathogens isolated from patients hospitalized with suspected pneumonia in Latin America: Frequency of occurrence and antimicrobial susceptibility profile: Results from the sentry antimicrobial surveillance program (1997–2000). Diag. Microbiol. Infect. Dis. 2002, 44, 301–311. [Google Scholar]
- Chi, H.; Chang, K.Y.; Chang, H.C.; Chiu, N.C.; Huang, F.Y. Infections associated with in dwelling ventriculostomy catheters in a teaching hospital. Int. J. Infect. Dis. 2010, 14, e216–219. [Google Scholar]
- Branski, L.K.; Al-Mousawi, A.; Rivero, H.; Jeschke, M.G.; Sanford, A.P.; Herndon, D.N. Emerging infections in burns. Surgical Infect. 2009, 10, 389–397. [Google Scholar]
- Walker, T.S.; Bais, H.P.; Deziel, E.; Schweizer, H.P.; Rahme, L.G.; Fall, R.; Vivanco, J.M. Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol. 2004, 134, 320–331. [Google Scholar]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clinical Invest. 2002, 109, 317–325. [Google Scholar]
- Erickson, D.L.; Endersby, R.; Kirkham, A.; Stuber, K.; Vollman, D.D.; Rabin, H.R.; Mitchell, I.; Storey, D.G. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 2002, 70, 1783–1790. [Google Scholar]
- Smith, R.S.; Harris, S.G.; Phipps, R.; Iglewski, B. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 2002, 184, 1132–1139. [Google Scholar]
- Ueda, A.; Wood, T.K. Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase Tpba (PA3885). PLoS Pathog. 2009. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. In M100-S22; CLSI: Wayne, PA, USA, 2012.
- Ee, R.; Lim, Y.L.; Tee, K.K.; Yin, W.F.; Chan, K.G. Quorum sensing activity of Serratia fonticola strain RB-25 isolated from an ex-landfill site. Sensors 2014, 14, 5136–5146. [Google Scholar]
- Robson Ee, H.-J.; Yin, W.F.; Chan, K.G. Pandoraea sp. RB-44, a novel quorum sensing soil bacterium. Sensors 2013, 13, 14121–14132. [Google Scholar]
- Ngeow, Y.; Cheng, H.; Chen, J.; Yin, W.-F.; Chan, K.-G. Short chain N-acyl homoserine lactone production by clinical multidrug resistant Klebsiella pneumoniae strain CSG20. Sensors 2013, 13, 15242–15251. [Google Scholar]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acyl homoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar]
- Winson, M.K.; Swift, S.; Fish, L.; Throup, J.P.; Jorgensen, F.; Chhabra, S.R.; Bycroft, B.W.; Williams, P.; Stewart, G.S. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 1998, 163, 185–192. [Google Scholar]
- Chen, J.; Koh, C.-L.; Sam, C.-K.; Yin, W.-F.; Chan, K.-G. Short chain N-acyl homoserine lactone production by soil isolate Burkholderia sp. strain A9. Sensors 2013, 13, 13217–13227. [Google Scholar]
- Lau, Y.; Sulaiman, J.; Chen, J.; Yin, W.-F.; Chan, K.-G. Quorum sensing activity of Enterobacter asburiae isolated from lettuce leaves. Sensors 2013, 13, 14189–14199. [Google Scholar]
- Chong, T.-M.; Koh, C.-L.; Sam, C.-K.; Choo, Y.-M.; Yin, W.-F.; Chan, K.-G. Characterization of quorum sensing and quorum quenching soil bacteria isolated from Malaysian tropical montane forest. Sensors 2012, 12, 4846–4859. [Google Scholar]
- Tan, L.Y.; Yin, W.F.; Chan, K.G. Silencing quorum sensing through extracts of Melicope lunu-ankenda. Sensors 2012, 12, 4339–4351. [Google Scholar]
- Priya, K.; Yin, W.F.; Chan, K.G. Anti-quorum sensing activity of the traditional chinese herb, Phyllanthus amarus. Sensors 2013, 13, 14558–14569. [Google Scholar]
- Mellmann, A.; Cloud, J.; Maier, T.; Keckevoet, U.; Ramminger, I.; Iwen, P.; Dunn, J.; Hall, G.; Wilson, D.; Lasala, P.; et al. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J. Clin. Microbiol. 2008, 46, 1946–1954. [Google Scholar]
- Wensing, A.; Zimmermann, S.; Geider, K. Identification of the corn pathogen Pantoea stewartii by mass spectrometry of whole-cell extracts and its detection with novel PCR primers. Appl. Environ. Microbiol. 2010, 76, 6248–6256. [Google Scholar]
- Fountoulakis, M. Proteomics: Current technologies and applications in neurological disorders and toxicology. Amino Acids 2001, 21, 363–381. [Google Scholar]
- Dworzanski, J.P.; Snyder, A.P. Classification and identification of bacteria using mass spectrometry-based proteomics. Expert Rev. Proteomics 2005, 2, 863–878. [Google Scholar]
- Da Silva Filho, L.V.; Tateno, A.F.; Martins, K.M.; Azzuz Chernishev, A.C.; Garcia Dde, O.; Haug, M.; Meisner, C.; Rodrigues, J.C.; Doring, G. The combination of PCR and serology increases the diagnosis of Pseudomonas aeruginosa colonization/infection in cystic fibrosis. Pediatr. Pulmo. 2007, 42, 938–944. [Google Scholar]
- Toder, D.S.; Gambello, M.J.; Iglewski, B.H. Pseudomonas aeruginosa LasA: A second elastase under the transcriptional control of lasR. Mol. Microbiol. 1991, 5, 2003–2010. [Google Scholar]
- Pearson, J.P.; Gray, K.M.; Passador, L.; Tucker, K.D.; Eberhard, A.; Iglewski, B.H.; Greenberg, E.P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sc. USA 1994, 91, 197–201. [Google Scholar]
- O'Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sc. USA 2013, 110, 17981–17986. [Google Scholar]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar]
- Smith, R.S.; Iglewski, B.H. Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J. Clin. Invest. 2003, 112, 1460–1465. [Google Scholar]

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, H.J.; Ee, R.; Cheong, Y.M.; Tan, W.-S.; Yin, W.-F.; Chan, K.-G. Detection of Quorum Sensing Activity in the Multidrug-Resistant Clinical Isolate Pseudomonas aeruginosa Strain GB11. Sensors 2014, 14, 12511-12522. https://doi.org/10.3390/s140712511
Cheng HJ, Ee R, Cheong YM, Tan W-S, Yin W-F, Chan K-G. Detection of Quorum Sensing Activity in the Multidrug-Resistant Clinical Isolate Pseudomonas aeruginosa Strain GB11. Sensors. 2014; 14(7):12511-12522. https://doi.org/10.3390/s140712511
Chicago/Turabian StyleCheng, Huey Jia, Robson Ee, Yuet Meng Cheong, Wen-Si Tan, Wai-Fong Yin, and Kok-Gan Chan. 2014. "Detection of Quorum Sensing Activity in the Multidrug-Resistant Clinical Isolate Pseudomonas aeruginosa Strain GB11" Sensors 14, no. 7: 12511-12522. https://doi.org/10.3390/s140712511