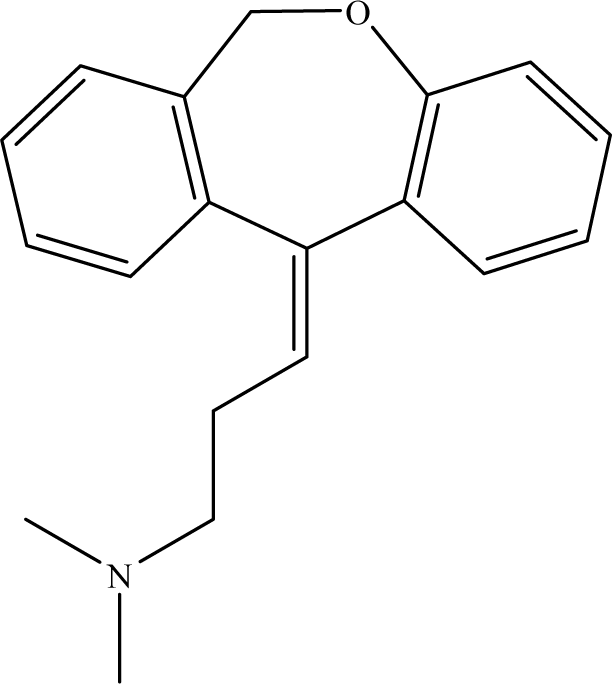
A Novel Electrochemical Sensor for Probing Doxepin Created on a Glassy Carbon Electrode Modified with Poly(4-Amino- benzoic Acid)/Multi-Walled Carbon Nanotubes Composite Film
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Apparatus
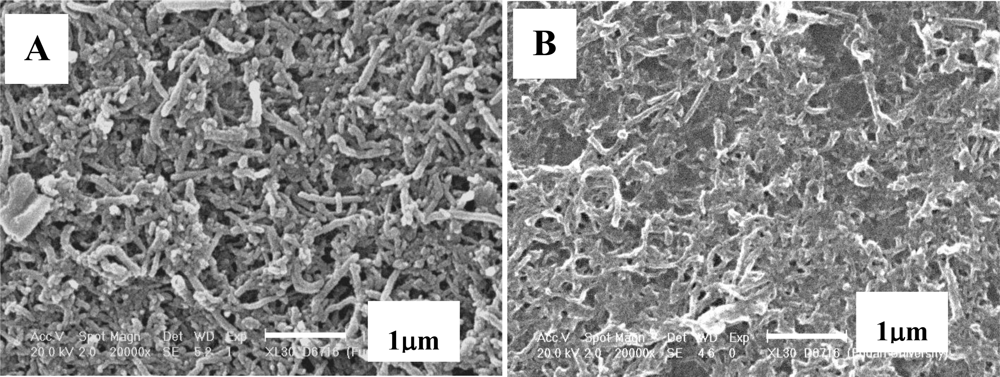
2.3. Fabrication of the poly(4-ABA)/MWNTs/GCE
3. Results and Discussion
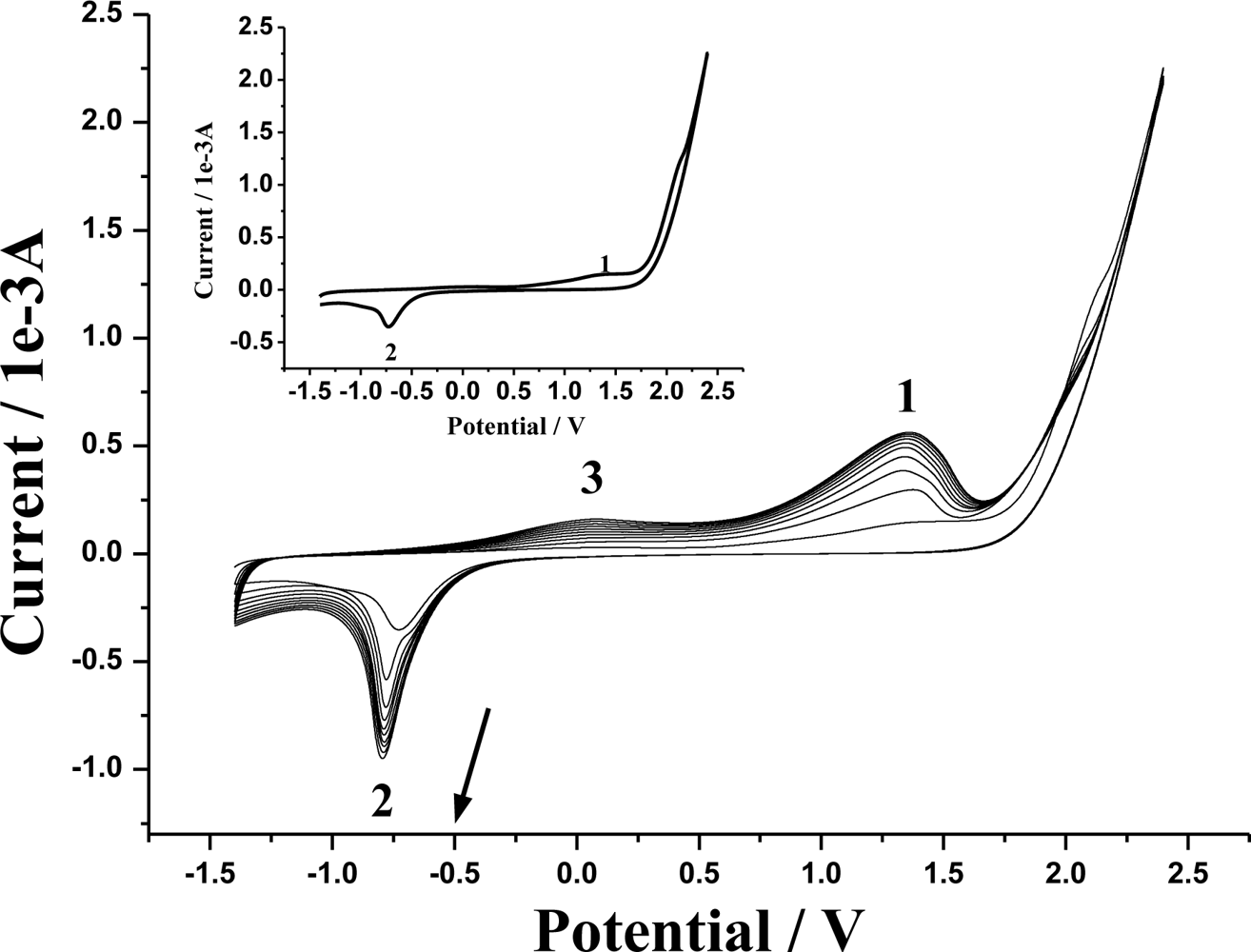
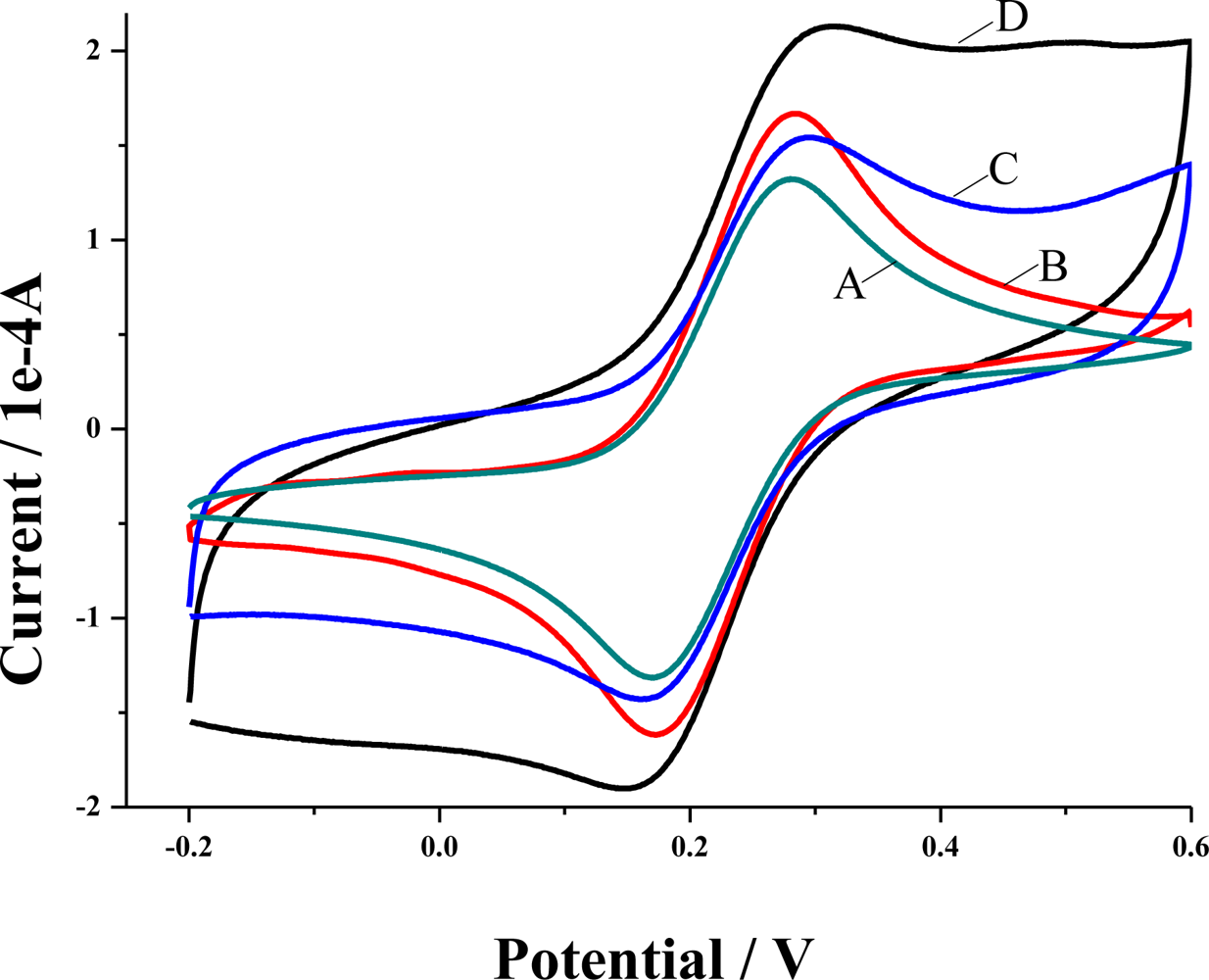
3.1. Cyclic voltammograms of electropolymerization of 4-ABA and characterization of the modified GCE
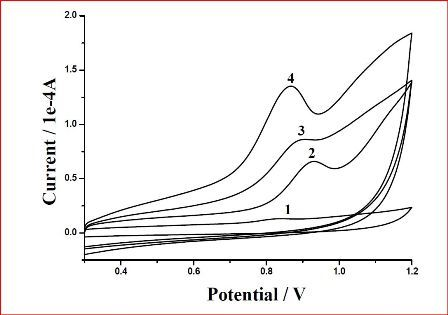
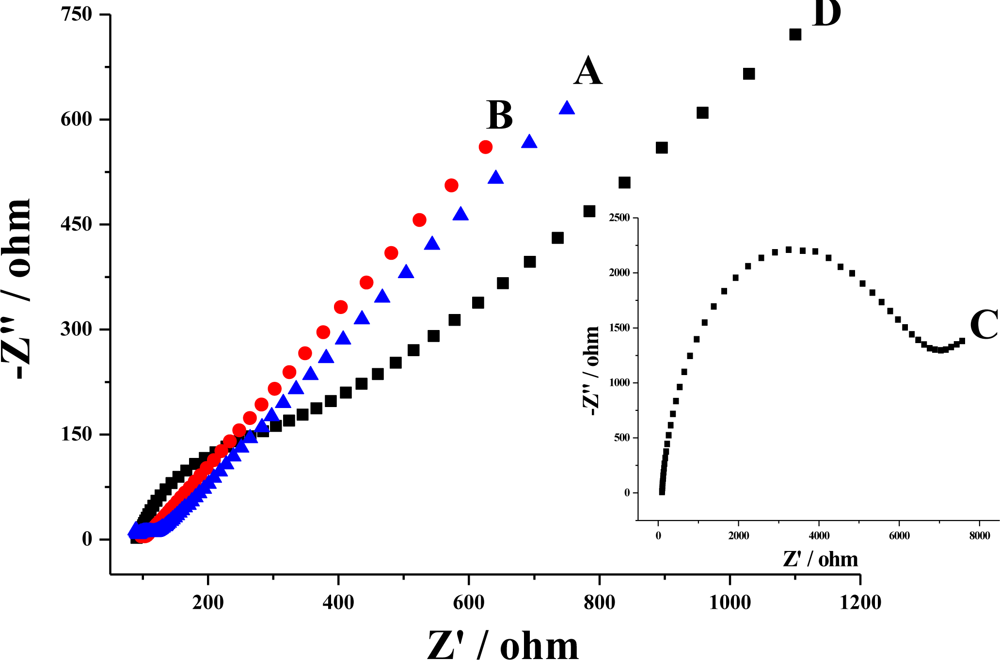
3.2. Electrochemical responses of the poly(4-ABA)/MWNTs/GCE
3.3. Effect of scan rate
3.4. Optimization of the analytical conditions
3.4.1. Influence of amount of MWNTs
3.4.2. Influence of electropolymerization conditions
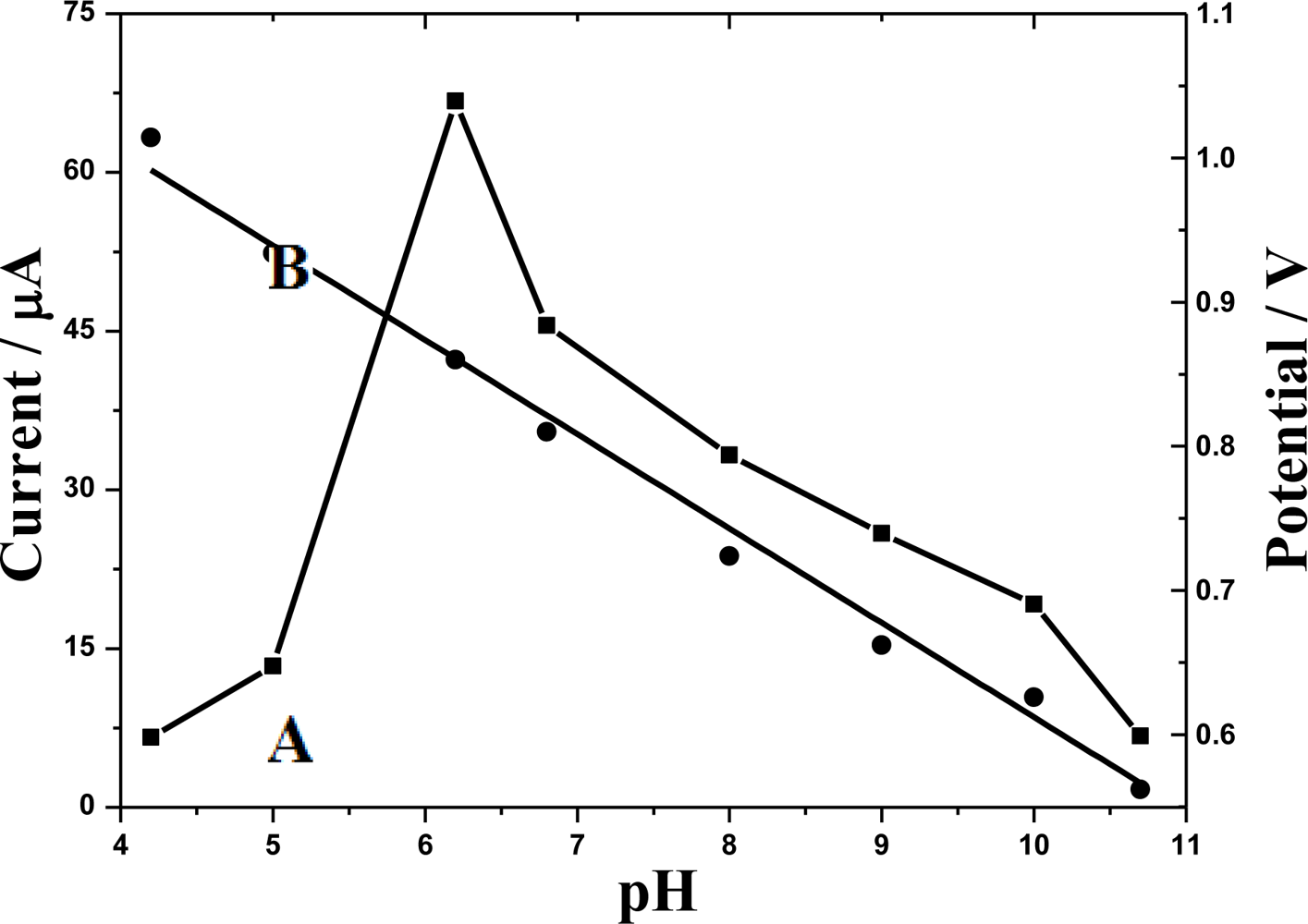
3.4.3. Influence of pH and buffer solution
3.4.4. Influence of accumulation time and potential
3.5. Calibration curve
3.6. The reproducibility, regeneration, stability and selectivity of poly(4-ABA)/MWNTs/GCE
3.7. Applications
4. Conclusions
sensors-10-08398-s001.pdf
Acknowledgments
References
- Chlobowska, Z; Chudzikiewicz, E; Koscienlniak, P; Piekoszewski, W; Stolarz, A. Application of Gas Chromatography with NPD Detection to the Analysis of Tricyclic Antidepressants in Blood. Chem. Anal 2003, 48, 255–263. [Google Scholar]
- Uddin, MN; Samanidou, VF; Papadoyannis, IN. Development and Validation of an HPLC Method for the Determination of Benzodiazepines and Tricyclic Antidepressants in Biological Fluids after Sequential Spe. J. Sep. Sci 2008, 31, 2358–2370. [Google Scholar]
- Ruiz-Angel, MJ; Carda-Broch, S; Simo-Alfonso, EF; Garcia-Alvarez-Coque, MC. Optimised Procedures for the Reversed-Phase Liquid Chromatographic Analysis of Formulations Containing Tricyclic Antidepressants. J. Pharm. Biomed. Anal 2003, 32, 71–84. [Google Scholar]
- Maslanka, A; Krzek, J. Densitometric High Performance Thin-Layer Chromatography Identification and Quantitative Analysis of Psychotropic Drugs. J. AOAC Int 2005, 88, 70–79. [Google Scholar]
- Gronewold, A; Dettling, A; Haffner, HT; Skopp, G. Doxepin and Nordoxepin Concentrations in Body Fluids and Tissues in Doxepin Associated Deaths. Forensic. Sci. Int 2009, 190, 74–79. [Google Scholar]
- Hostetter, AL; Stowe, ZN; Cox, M; Ritchie, JC. A Novel System for the Determination of Antidepressant Concentrations in Human Breast Milk. Ther. Drug Monit 2004, 26, 47–52. [Google Scholar]
- Ma, Q; Chen, M; Shi, ZG; Feng, YQ. Preparation of a Poly(N-Isopropylacrylamide-Co-Ethylene Dimethacrylate) Monolithic Capillary and Its Application for in-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. J. Sep. Sci 2009, 32, 2592–2600. [Google Scholar]
- Hummel, D; Loffler, D; Fink, G; Ternes, TA. Simultaneous Determination of Psychoactive Drugs and Their Metabolites in Aqueous Matrices by Liquid Chromatography Mass Spectrometry. Environ. Sci. Technol 2006, 40, 7321–7328. [Google Scholar]
- Kou, HS; Chen, CC; Huang, YH; Ko, WK; Wu, HL; Wu, SM. Method for Simultaneous Determination of Eight Cyclic Antidepressants by Cyclodextrin-Modified Capillary Zone Electrophoresis: Applications in Pharmaceuticals. Anal. Chim. Acta 2004, 525, 23–30. [Google Scholar]
- Greenway, GM; Dolman, SJL. Analysis of Tricyclic Antidepressants Using Electrogenerated Chemiluminescence. Analyst 1999, 124, 759–762. [Google Scholar]
- Wu, CK; Feng, SL; Jing, F. Spectrometric Study on the Interaction of Doxepin Hydrochloride and Fast Green and Its Application. Spectrosc. Spectr. Anal 2007, 27, 2490–2493. [Google Scholar]
- Georgiou, ME; Georgiou, CA; Koupparis, MA. Rapid Automated Spectrophotometric Competitive Complexation Studies of Drugs with Cyclodextrins Using the Flow Injection Gradient Technique: Tricyclic Antidepressant Drugs with Alpha-Cyclodextrin. Analyst 1999, 124, 391–396. [Google Scholar]
- Acedo-Valenzuela, MI; Galeano-Diaz, T; Mora-Diez, N; Silva-Rodriguez, A. Response Surface Methodology for the Optimisation of Flow-Injection Analysis with in Situ Solvent Extraction and Fluorimetric Assay of Tricyclic Antidepressants. Talanta 2005, 66, 952–960. [Google Scholar]
- Chodkowski, J; Chrzanowski, A; Gralewskaludwicka, D. Polarographic-Determination of Doxepin Hydrochloride. Chem. Anal 1992, 37, 629–633. [Google Scholar]
- Hopkala, H; Zareba, S. Doxepin Ion-Selective Membrane Electrode, Preparation and Use in Pharmaceutical Analysis. Chem. Anal 1996, 41, 413–417. [Google Scholar]
- Ivandini, TA; Sarada, BV; Terashima, C; Rao, TN; Tryk, DA; Ishiguro, H; Kubota, Y; Fujishima, A. Electrochemical Detection of Tricyclic Antidepressant Drugs by HPLC Using Highly Boron-Doped Diamond Electrodes. J. Electroanal. Chem 2002, 521, 117–126. [Google Scholar]
- Huang, F; Peng, YY; Jin, GY; Zhang, S; Kong, JL. Sensitive Detection of Haloperidol and Hydroxyzine at Multi-Walled Carbon Nanotubes-Modified Glassy Carbon Electrodes. Sensors 2008, 8, 1879–1889. [Google Scholar]
- Jin, GY; Zhang, YH; Cheng, WX. Poly(P-Aminobenzene Sulfonic Acid)-Modified Glassy Carbon Electrode for Simultaneous Detection of Dopamine and Ascorbic Acid. Sens. Actuator. B-Chem 2005, 107, 528–534. [Google Scholar]
- Huang, F; Jin, GY; Liu, Y; Kong, JL. Sensitive Determination of Phenylephrine and Chlorprothixene at Poly(4-Aminobenzene Sulfonic Acid) Modified Glassy Carbon Electrode. Talanta 2008, 74, 1435–1441. [Google Scholar]
- Jin, GY; Huang, F; Kong, JL. Sensitive Determination of Clomipramine at Poly-ABSA/Pt Nano-Clusters Modified Glassy Carbon Electrode. Anal. Lett 2007, 40, 3392–3404. [Google Scholar]
- Jin, GY; Huang, F; Li, W; Yu, SN; Zhang, S; Kong, JL. Sensitive Detection of Trifluoperazine Using a Poly-ABSA/SWNTs Film-Modified Glassy Carbon Electrode. Talanta 2008, 74, 815–820. [Google Scholar]
- Xu, F; Gao, MN; Wang, L; Shi, GY; Zhang, W; Jin, LT; Jin, JY. Sensitive Determination of Dopamine on Poly(Aminobenzoic Acid) Modified Electrode and the Application toward an Experimental Parkinsonian Animal Model. Talanta 2001, 55, 329–336. [Google Scholar]
- Xu, F; Gao, MN; Shi, GY; Wang, L; Zhang, W; Xue, J; Jin, LT; Jin, JY. Simultaneous Detection of Monoamines in Rat Striatal Microdialysate at Poly(Para-Aminobenzoic Acid) Modified Electrode by High-Performance Liquid Chromatography. Anal. Chim. Acta 2001, 439, 239–246. [Google Scholar]
- Liu, JY; Cheng, L; Li, BF; Dong, SJ. Covalent Modification of a Glassy Carbon Surface by 4-Aminobenzoic Acid and Its Application in Fabrication of a Polyoxometalates-Consisting Monolayer and Multilayer Films. Langmuir 2000, 16, 7471–7476. [Google Scholar]
- Bard, AJ; Faulkner, LR. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 1980; p. 595. [Google Scholar]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem 1979, 101, 19–28. [Google Scholar]
- Dell’Aquila, C. Separation of Tricyclic Antidepressants by Capillary Zone Electrophoresis with N,N,N′,N′-Tetramethyl-1,3-Butanediamine (TMBD) as an Effective Electrolyte Additive. J. Pharm. Biomed. Anal 2002, 30, 341–350. [Google Scholar]
- Chinese Pharmacopoeia Committee. Chinese Pharmacopoeia (Part II), 1st ed; Chemical Industry Press: Beijing, China, 2005; pp. 505–506. [Google Scholar]
- Li, JG; Zhao, FJ; Ju, HX. Simultaneous Determination of Psychotropic Drugs in Human Urine by Capillary Electrophoresis with Electrochemiluminescence Detection. Anal. Chim. Acta 2006, 575, 57–61. [Google Scholar]

| Sample number | Added (μg/mL) | Expected (μg/mL) | Found (μg/mL) | R.S.D.(%) | Recovery (%) | UV method (μg/mL) |
|---|---|---|---|---|---|---|
| 0 | 0 | 1.00 | 0.99 | 2.5 | 99.0 | 0.98 |
| 1 | 1.00 | 2.00 | 1.96 | 2.9 | 98.0 | 1.99 |
| 2 | 2.00 | 3.00 | 2.97 | 2.1 | 99.0 | 2.98 |
| 3 | 3.00 | 4.00 | 3.94 | 3.3 | 98.5 | 4.02 |
| 4 | 4.00 | 5.00 | 5.02 | 1.7 | 100.4 | 4.99 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, X.-L.; Huang, F.; Zhou, G.-L.; Zhang, S.; Kong, J.-L. A Novel Electrochemical Sensor for Probing Doxepin Created on a Glassy Carbon Electrode Modified with Poly(4-Amino- benzoic Acid)/Multi-Walled Carbon Nanotubes Composite Film. Sensors 2010, 10, 8398-8410. https://doi.org/10.3390/s100908398
Xu X-L, Huang F, Zhou G-L, Zhang S, Kong J-L. A Novel Electrochemical Sensor for Probing Doxepin Created on a Glassy Carbon Electrode Modified with Poly(4-Amino- benzoic Acid)/Multi-Walled Carbon Nanotubes Composite Film. Sensors. 2010; 10(9):8398-8410. https://doi.org/10.3390/s100908398
Chicago/Turabian StyleXu, Xiao-Li, Fei Huang, Guo-Liang Zhou, Song Zhang, and Ji-Lie Kong. 2010. "A Novel Electrochemical Sensor for Probing Doxepin Created on a Glassy Carbon Electrode Modified with Poly(4-Amino- benzoic Acid)/Multi-Walled Carbon Nanotubes Composite Film" Sensors 10, no. 9: 8398-8410. https://doi.org/10.3390/s100908398