Development of a Fully Automated Flow Injection Analyzer Implementing Bioluminescent Biosensors for Water Toxicity Assessment
Abstract
:1. Introduction
2. Experimental Section
2.1. V. fischeri Culture
2.2. Chemicals
3. Results and Discussion
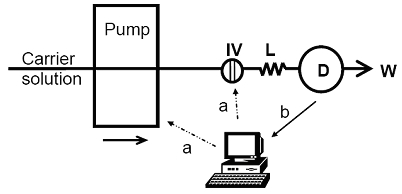
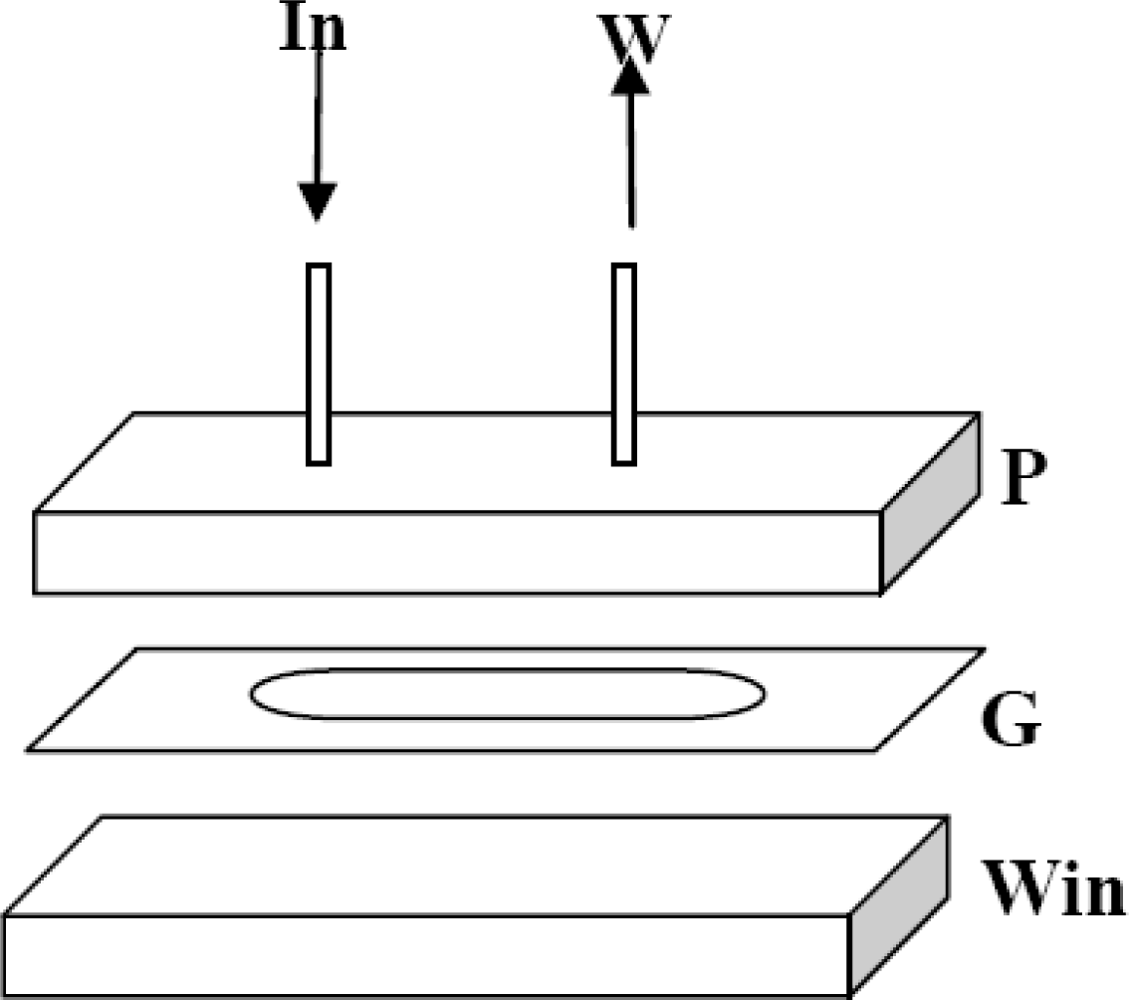
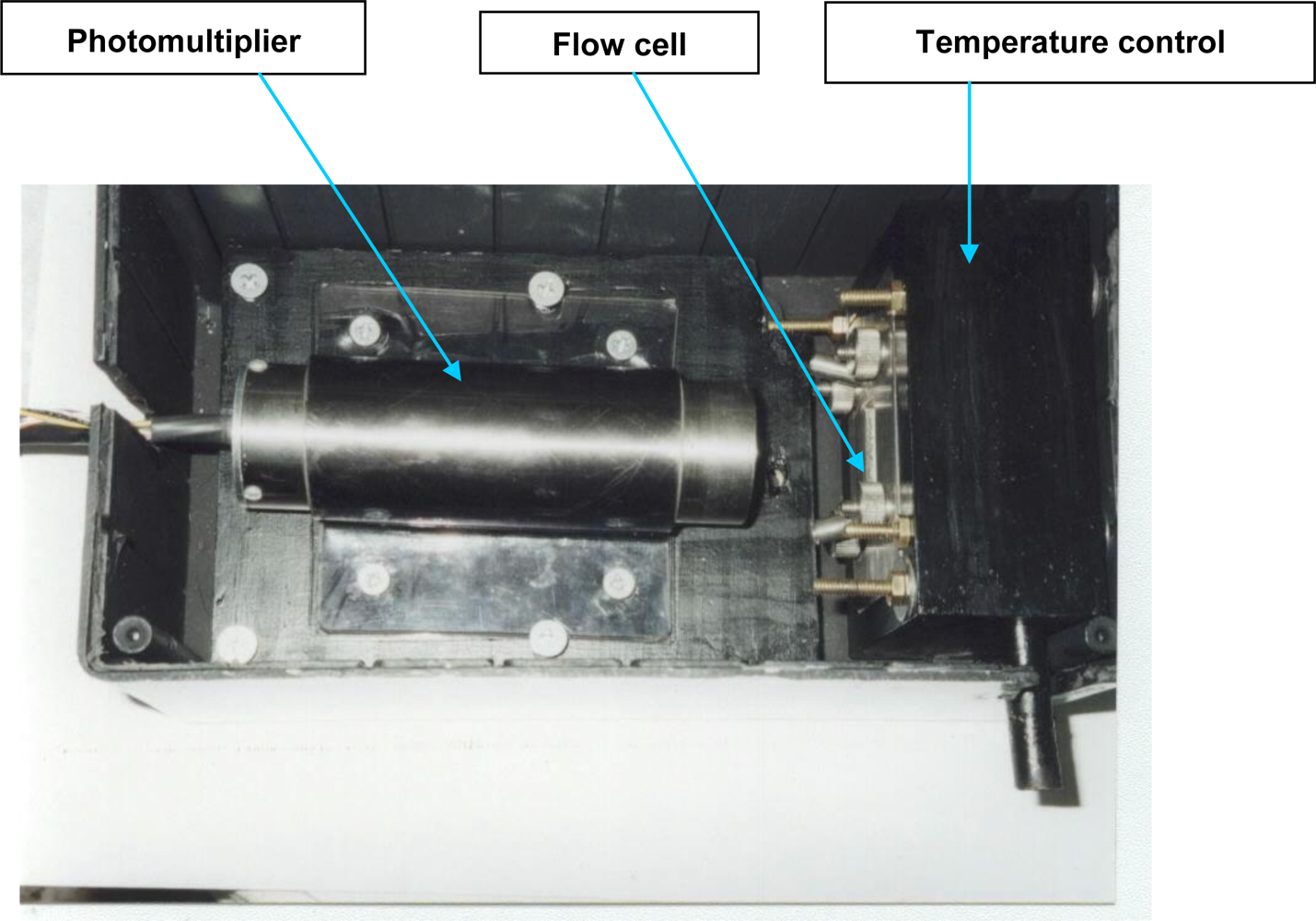
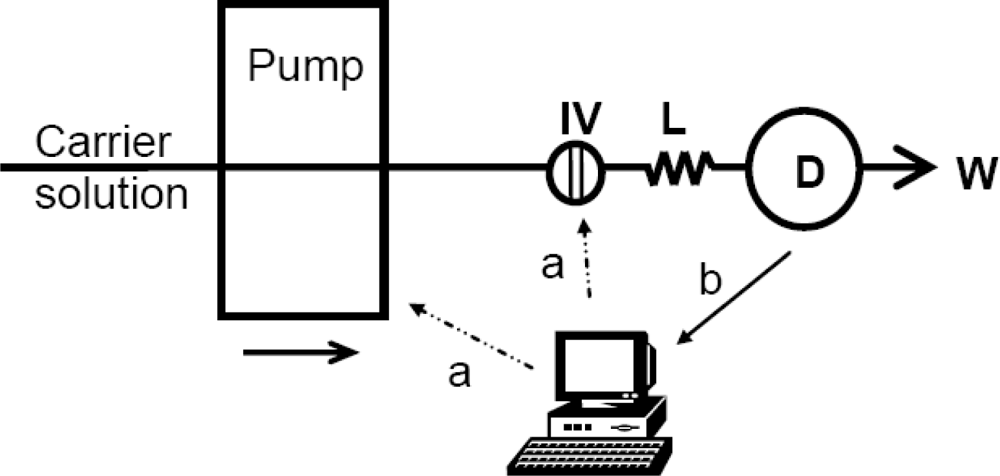
3.1. Detector Unit Development
3.2. Flow Injection Analyzer Development and Optimisation
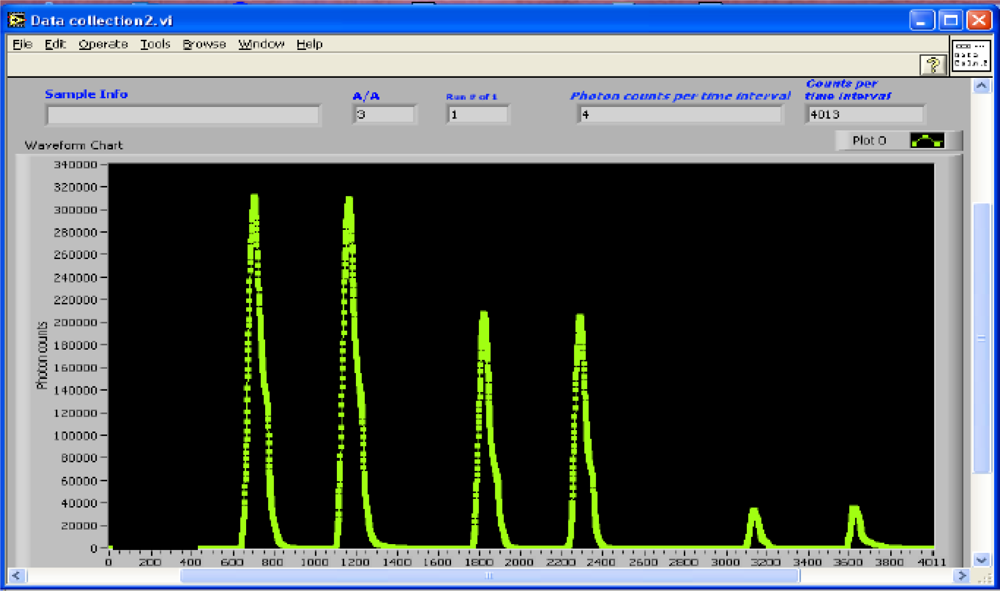
3.3. Software
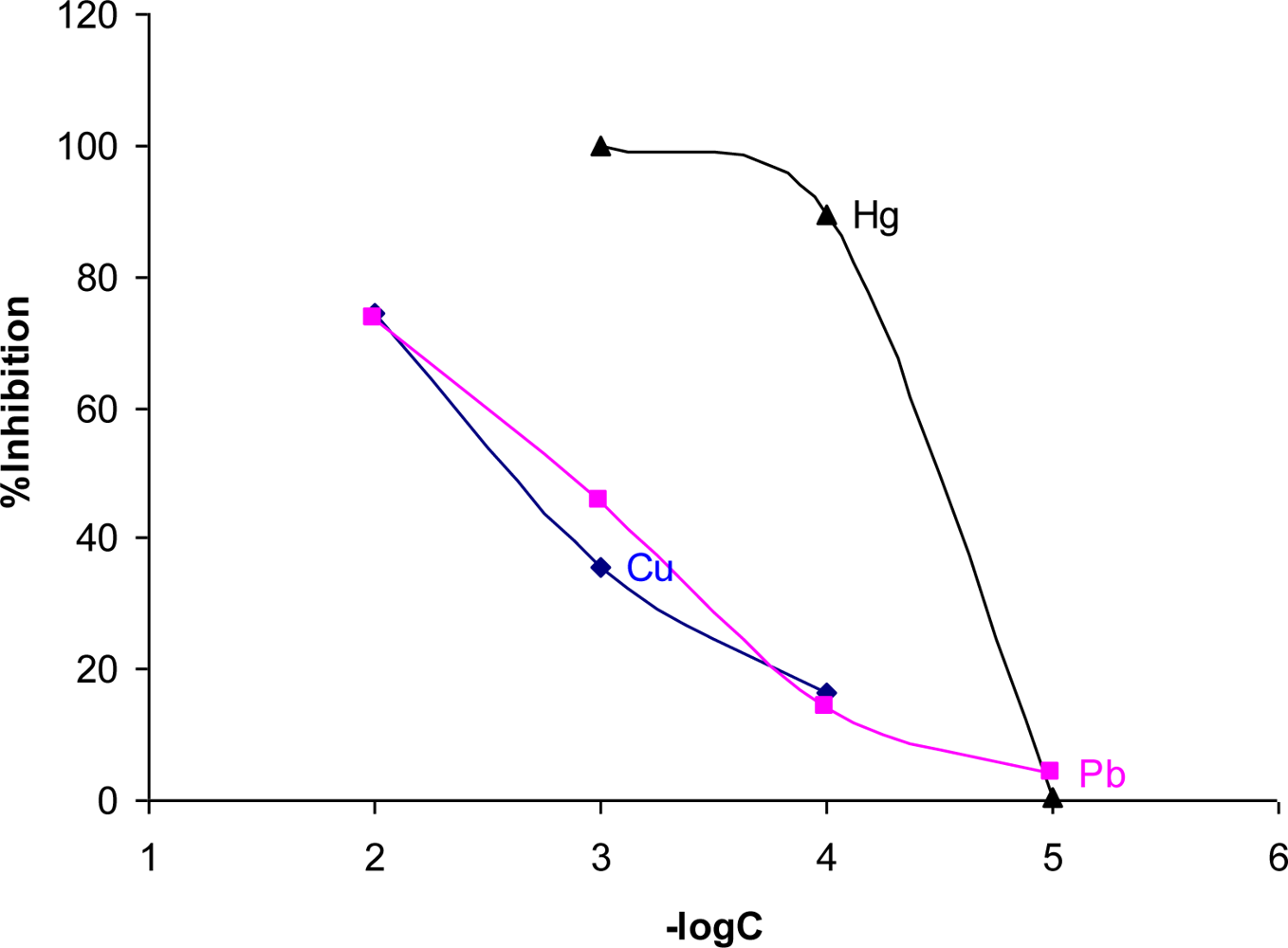
3.4. Assessment of Heavy Metals Toxicity
4. Conclusions
Acknowledgments
References and Notes
- Dewhurst, RE; Wheeler, JR; Chummun, KS; Mather, JD; Callaghan, A; Crane, M. The comparison of rapid bioassays for the assessment of urban groundwater quality. Chemosphere 2002, 47, 547–554. [Google Scholar]
- Forget, G; Sanchez-Bain, A; Arkhipchuk, V; Beauregard, T; Blaise, C; Castillo, G; Castillo, LE; Diaz-Baez, MC; Pica-Granados, Y; Ronco, A; Srivastava, RC; Dutka, BJ. Preliminary data of a single-blind, multicountry trial of six bioassays for water toxicity monitoring. Environ. Toxicol 2000, 15, 362–369. [Google Scholar]
- Deryabin, DG; Karimov, IF. Simultaneous evaluation of chemiluminescence and bioluminescence in a phagocytic system. Bull. Exp. Biol. Med-Engl. Tr 2009, 147, 349–352. [Google Scholar]
- Farre, M; Barcelo, D. Toxicity testing of wastewater and sewage sludge by biosensors, bioassays and chemical analysis. Trac-Trend. Anal. Chem 2003, 22, 299–310. [Google Scholar]
- El-Alawi, YS; Huang, XD; Dixon, DG; Greenberg, BM. Quantitative structure-activity relationship for the photoinduced toxicity of polycyclic aromatic hydrocarbons to the luminescent bacteria Vibrio fischeri. Environ. Toxicol. Chem 2002, 21, 2225–2232. [Google Scholar]
- D'Souza, SF. Microbial biosensors. Biosens. Bioelectron 2001, 16, 337–353. [Google Scholar]
- Divritsioti, MH; Karalemas, ID; Georgiou, CA; Papastathopoulos, DS. Flow Injection analysis system for l-lysine estimation in foodstuffs using a biosensor based on lysine oxidase immobilization on a gold-poly (m-phenylenediamine) electrode. Anal. Lett 2003, 36, 1939–1963. [Google Scholar]
- Liu, XM; Germaine, KJ; Ruan, D; Dowling, D. Whole cell fluorescent biosensors for bioavailability and biodegradation of polychlorinated biphenyls. Sensors 2010, 10, 1377–1398. [Google Scholar]
- Beyersdorf-Radeck, B; Riedel, K; Karlson, U; Bachmann, TT; Schmid, RD. Screening of xenobiotic compounds degrading microorganisms using biosensor techniques. Microbiol. Res 1998, 153, 239–245. [Google Scholar]
- Petanen, T; Romantschuk, M. Use of bioluminescent bacterial sensors as an alternative method for measuring heavy metals in soil extracts. Anal. Chim. Acta 2002, 456, 55–61. [Google Scholar]
- Liu, Q; Cai, H; Xiao, L; Li, R; Yang, M; Wang, P. Embryonic stem cells biosensor and its application in drug analysis and toxin detection. IEEE Sensors J 2007, 7, 1625–1631. [Google Scholar]
- Peters, M; Vaillancourt, F; Xeroux, M; Valiquette, M; Scott, C. Comparing label-free biosensors for pharmacological screening with cell-based functional assays. Assay Drug Dev. Technol 2010, 8, 219–227. [Google Scholar]
- Baeumner, AJ. Biosensors for environmental pollutants and food contaminants. Anal. Bioanal. Chem 2003, 377, 434–445. [Google Scholar]
- Melidis, P; Vaiopoulou, E; Aivasidis, A. Development and implementation of microbial sensors for efficient process control in wastewater treatment plants. Bioprocess Biosyst. Eng 2008, 31, 277–282. [Google Scholar]
- Minioti, KS; Georgiou, CA. Comparison of different tests used in mapping the Greek virgin olive oil production for the determination of its total antioxidant capacity. Grasas Aceites 2010, 61, 45–51. [Google Scholar]
- Mimendia, A; Legin, A; Mercoci, A; del Valle, M. Use of sequential injection analysis to construct a potentiometric electronic tongue: Application to the multidetermination of heavy metals. Sens Actuat B-Chem 2010, 420–426. [Google Scholar]


© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Komaitis, E.; Vasiliou, E.; Kremmydas, G.; Georgakopoulos, D.G.; Georgiou, C. Development of a Fully Automated Flow Injection Analyzer Implementing Bioluminescent Biosensors for Water Toxicity Assessment. Sensors 2010, 10, 7089-7098. https://doi.org/10.3390/s100807089
Komaitis E, Vasiliou E, Kremmydas G, Georgakopoulos DG, Georgiou C. Development of a Fully Automated Flow Injection Analyzer Implementing Bioluminescent Biosensors for Water Toxicity Assessment. Sensors. 2010; 10(8):7089-7098. https://doi.org/10.3390/s100807089
Chicago/Turabian StyleKomaitis, Efstratios, Efstathios Vasiliou, Gerasimos Kremmydas, Dimitrios G. Georgakopoulos, and Constantinos Georgiou. 2010. "Development of a Fully Automated Flow Injection Analyzer Implementing Bioluminescent Biosensors for Water Toxicity Assessment" Sensors 10, no. 8: 7089-7098. https://doi.org/10.3390/s100807089