A Novel pH-dependent Drift Improvement Method for Zirconium Dioxide Gated pH-Ion Sensitive Field Effect Transistors
Abstract
:1. Introduction
2. Experimental
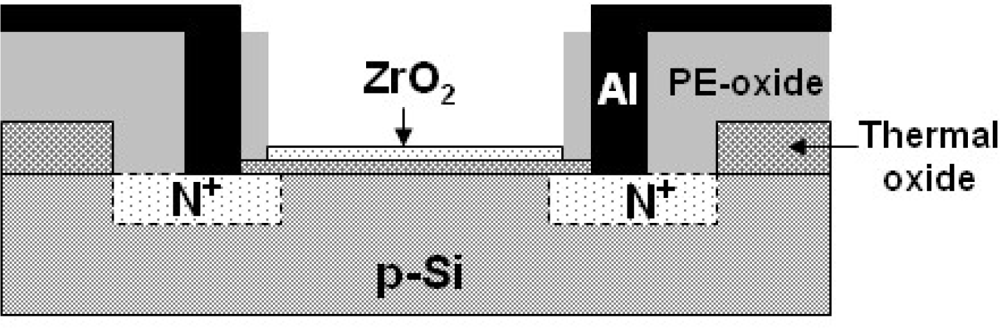
2.1. Device Fabrication
- Standard RCA clean for 4-inchp-type and n-type silicon wafers
- Wet oxidation growth for silicon dioxide (600 nm)
- Defining of Source/Drain areas with mask I and wet etching of silicon dioxide by Buffered Oxide Etching (BOE)
- Thermal growth of silicon dioxide as screen oxide (30 nm)
- Phosphorus or boron ions implantation and post annealing at 950 °C
- Plasma Enhanced Chemical Vapor Deposition (PECVD) deposition of silicon dioxide as passivation layer
- Defining of contact hole and gate region with mask II and wet etching of silicon dioxide by BOE
- Dry oxidation of gate oxide (30 nm)
- DC sputtering of ZrO2 (30 nm) and post annealing at 600 °C
- Defining of gate region and wet etching of oxide by BOE
- Aluminum sputtering with hard contact mask (500 nm)
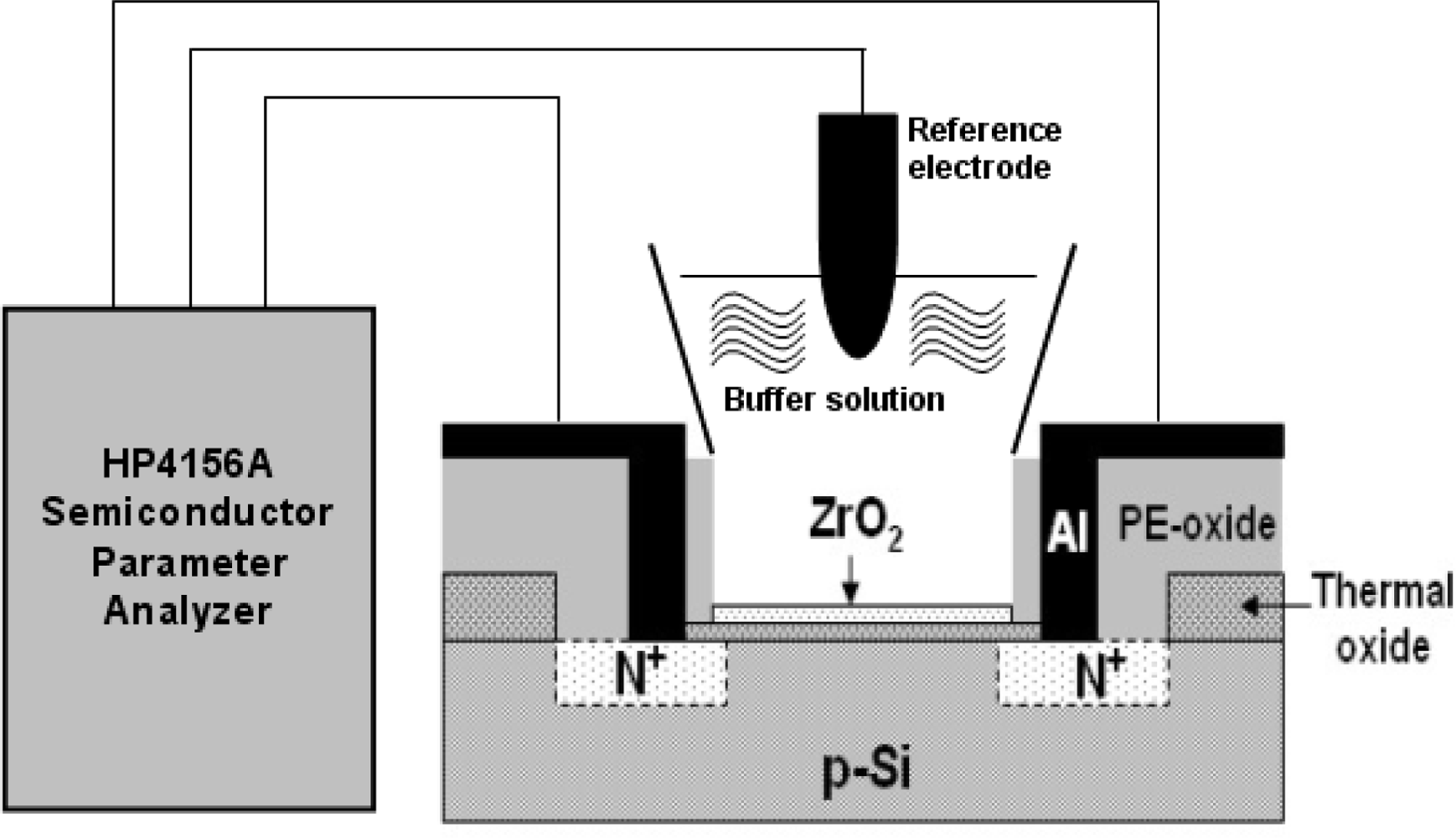
2.2. Packaging and Measurement
3. Results and Discussion
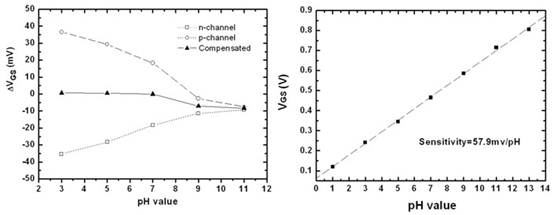
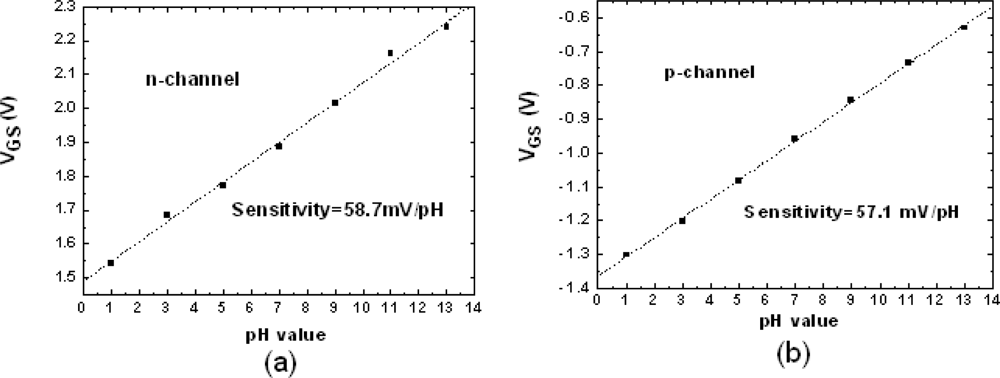
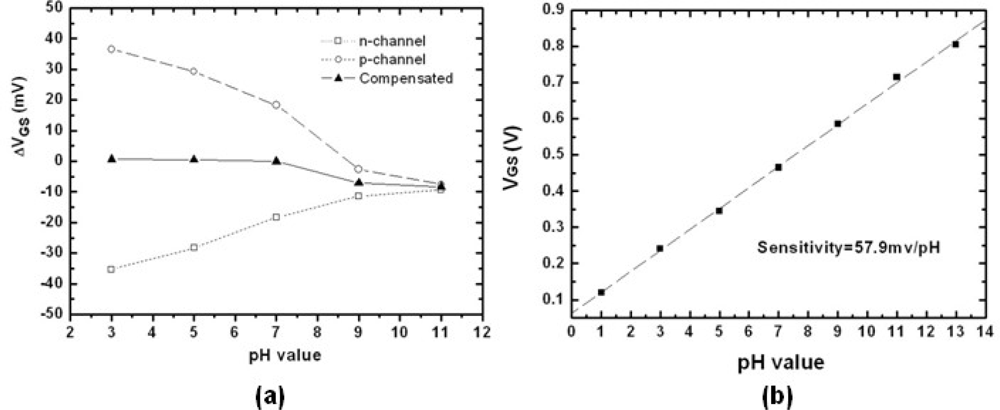
3.1. pH Sensitivity of n-channel and p-channel ZrO2 Gate ISFETs
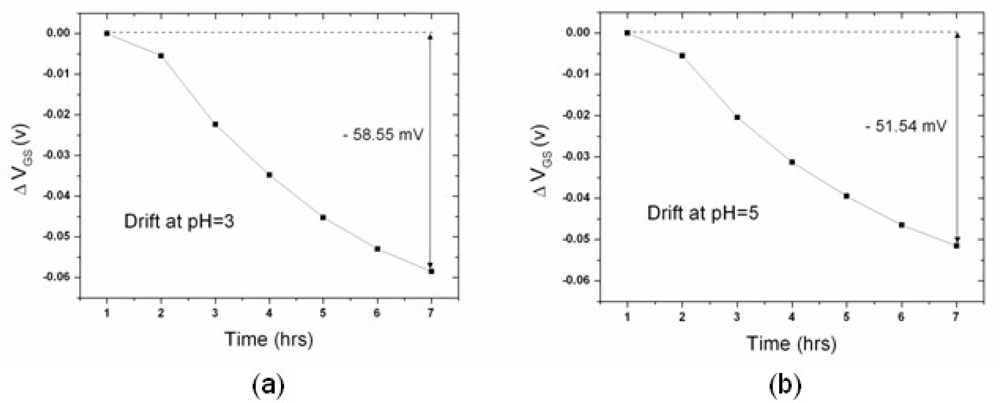
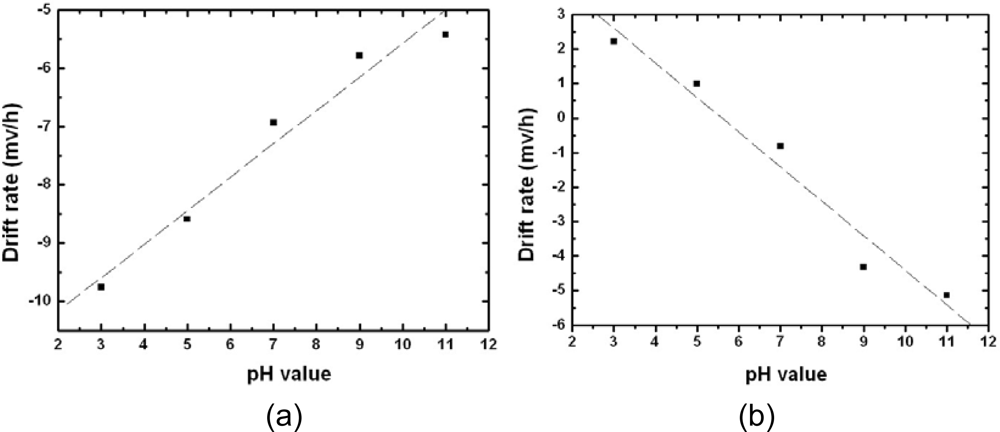
3.2. Drift Rate of n-type and p-type ZrO2 Gate ISFETs
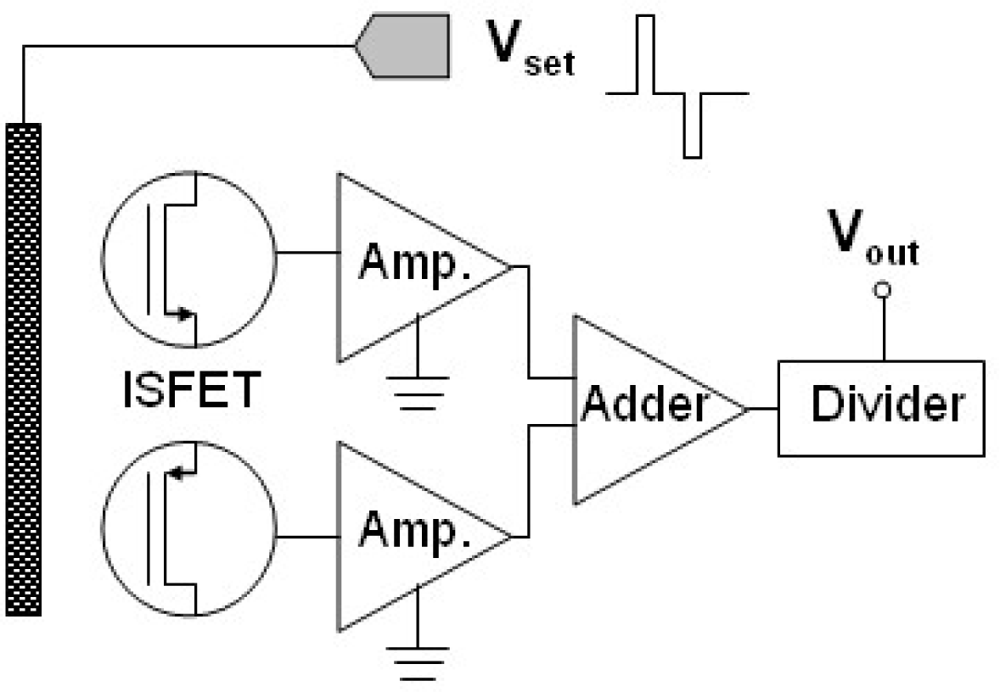
3.3. Drift Rate and pH Sensitivity of Combined n-channel and p-channel ZrO2 Gate ISFETs
4. Conclusions
Acknowledgments
References and Notes
- Bergveld, P. Development of an ion-sensitive solid-state device for neuro-physiological measurements. IEEE Trans. Biomed Eng 1970, BME 17, 70–71. [Google Scholar]
- Schoning, M.J.; Brinkmann, D.; Rolka, D.; Demuth, C.; Poghossian, A. CIP (cleaning-in-place) suitable “non-glass” pH sensor based on a Ta2O5-gate EIS structure. Sens. Actuat. B 2005, 111–112, 423–429. [Google Scholar]
- Bousse, L.; Bergveld, P. The role of buried OH sites in the response mechanism of inorganic-gate pH-sensitive ISFETs. Sens. Actuat 1984, 6, 65–78. [Google Scholar]
- Jamasb, S.; Collins, S.D.; Smith, R.L. A physical model for threshold voltage instability in Si3N4-gate H+-sensitive FET’s (pH-ISFET’s). IEEE Trans. Electron Devices 1998, 45, 1239–1245. [Google Scholar]
- Abe, H.; Esashi, M.; Matsuo, T. ISFET’s Using Inorganic Gate Thin Films. IEEE Trans. Electron Devices 1979, 26, 1939–1944. [Google Scholar]
- Topkar, A.; Lal, R. Effect of electrolyte exposure on silicon dioxide in electrolyte-oxide-semiconductor structures. Thin Solid Films 1993, 232, 265–270. [Google Scholar]
- Buck, R.P. Kinetics and Drift of Gate Voltages for Electrolyte-Bathed Chemically Sensitive Semiconductor Devices. IEEE Trans. Electron Devices 1982, 29, 108–115. [Google Scholar]
- Dun, Y.; Ya-dong, W.; Gui-hua, W. Time-dependent response characteristics of pH-sensitive ISFET. Sens. Actuat. B 1991, 3, 279–285. [Google Scholar]
- Esashi, M; Matsuo, T. Integrated Micro Multi Ion Sensor Using Field Effect of Semiconductor. IEEE Trans. Biomed Eng 1978, BME 25, 84–92. [Google Scholar]
- Chu-Neng, T.; Jung-Chuan, C.; Tai-Ping, S.; Shen-kan, H. Study on the Time-Dependent Slow Response of the Tin Oxide pH Electrode. IEEE Sensors J 2006, 6, 1243–1249. [Google Scholar]
- Jung-Chuan, C.; Yii Fang, W. Preparation and study on the drift and hysteresis properties of the tin oxide gate ISFET by the sol–gel method. Sens. Actuat. B 2002, 86, 58–62. [Google Scholar]
- Jung-Chuan, C.; Ching Nan, H. The hysteresis and drift effect of hydrogenated amorphous silicon for ISFET. Sens. Actuat. B 2000, 66, 181–183. [Google Scholar]
- Jung-Lung, C.; Shiun-Sheng, J.; Jung-Chuan, C.; Ying-Chung, C. Study on the temperature effect, hysteresis and drift of pH-ISFET devices based on amorphous tungsten oxide. Sens. Actuat. B 2001, 76, 624–628. [Google Scholar]
- Jung-Lung, C.; Jung-Chuan, C.; Ying-Chung, C.; Guo Shiang, L.; Chien-Chuan, C. Drift and Hysteresis Effects on AlN/SiO2 Gate pH Ion-Sensitive Field-Effect Transistor. Jpn. J. Appl. Phys 2003, 42, 4973–4977. [Google Scholar]
- Bousse, L.; Van Den Vlekkert, H.H.; De Rooij, N.F. Hysteresis in Al2O3-gate ISFETs. Sens. Actuat. B 1990, 2, 103–110. [Google Scholar]
- Hendrikse, J.; Olthuis, W.; Bergveld, P. A Drift Free Nernstian Irdium Oxide pH Sensor. IEEE International Conference on Solid-state Sensors and Actuators, Chicago, IL, USA, June 16–19, 1997; pp. 1367–1370.
- Hein, P.; Egger, P. Drift behaviour of ISFETs with Si3N4–SiO2 gate insulator. Sens. Actuat. B 1993, 13, 655–656. [Google Scholar]
- Bousse, L.; De Rooji, N.F.; Bergveld, P. Operation of chemically sensitive field-effect sensors as a function of the insulator-electrolyte interface. IEEE Trans. Electron Devices 1983, 30, 1263–1270. [Google Scholar]
- Poghossian, A.; Baade, A.; Emons, H.; Schoning, M.J. Application of ISFETs for pH measurement in rain droplets. Sens. Actuat. B 2001, 76, 634–638. [Google Scholar]
- Jamasb, S.; Collins, Scott D.; Smith, Rosemary L. A Physically-based Model for Drift in A1203-gate pH ISFET’s. IEEE International Conference on Solid-state Sensors and Actuators, Chicago, IL, USA, June 16–19, 1997; pp. 1379–1382.
- Matsuo, T.; Esashi, M. Methods of ISFET fabrication. Sens. Actuat. B 1981, 1, 77–96. [Google Scholar]
- Yu, Dun; Ya-dong, W.; Gui-hua, W. Time-dependent response characteristics of pH-sensitive ISFET. Sens. Actuat. B 1991, 3, 279–285. [Google Scholar]
- Van Hal, R.E.G.; Bergveld, P.; Engbersen, J.F.J. Fabrication and packaging of Mesa ISFETs. Sens. Mater 1996, 8, 455–468. [Google Scholar]
- Poghossian, A.; Schoning, M.J. Detecting Both Physical and (Bio-)Chemical Parameters by Means of ISFET Devices. Electroanalysis 2004, 16, 1863–1872. [Google Scholar]
- Kow-Ming, C.; Kuo-Yi, C.; Ting-Wei, C.; Chih-Tien, C. Characteristics of Zirconium Oxide Gate Ion-Sensitive Field-Effect Transistors. Jpn. J. Appl. Phys 2007, 46, 4333–4337. [Google Scholar]
- Van Hal, R.E.G.; Eijkel, J.C.T.; Bergveld, P. A novel description of ISFET sensitivity with the buffer capacity and double layer capacitance as key parameters. Sens. Actuat. B 1995, 24–25, 201–205. [Google Scholar]




© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/)
Share and Cite
Chang, K.-M.; Chang, C.-T.; Chao, K.-Y.; Lin, C.-H. A Novel pH-dependent Drift Improvement Method for Zirconium Dioxide Gated pH-Ion Sensitive Field Effect Transistors. Sensors 2010, 10, 4643-4654. https://doi.org/10.3390/s100504643
Chang K-M, Chang C-T, Chao K-Y, Lin C-H. A Novel pH-dependent Drift Improvement Method for Zirconium Dioxide Gated pH-Ion Sensitive Field Effect Transistors. Sensors. 2010; 10(5):4643-4654. https://doi.org/10.3390/s100504643
Chicago/Turabian StyleChang, Kow-Ming, Chih-Tien Chang, Kuo-Yi Chao, and Chia-Hung Lin. 2010. "A Novel pH-dependent Drift Improvement Method for Zirconium Dioxide Gated pH-Ion Sensitive Field Effect Transistors" Sensors 10, no. 5: 4643-4654. https://doi.org/10.3390/s100504643