An Optical Biosensor for Monitoring Antigen Recognition Based on Surface Plasmon Resonance Using Avidin-Biotin System
Abstract
:Introduction
Experimental
Reagents and Materials
SPR set-up
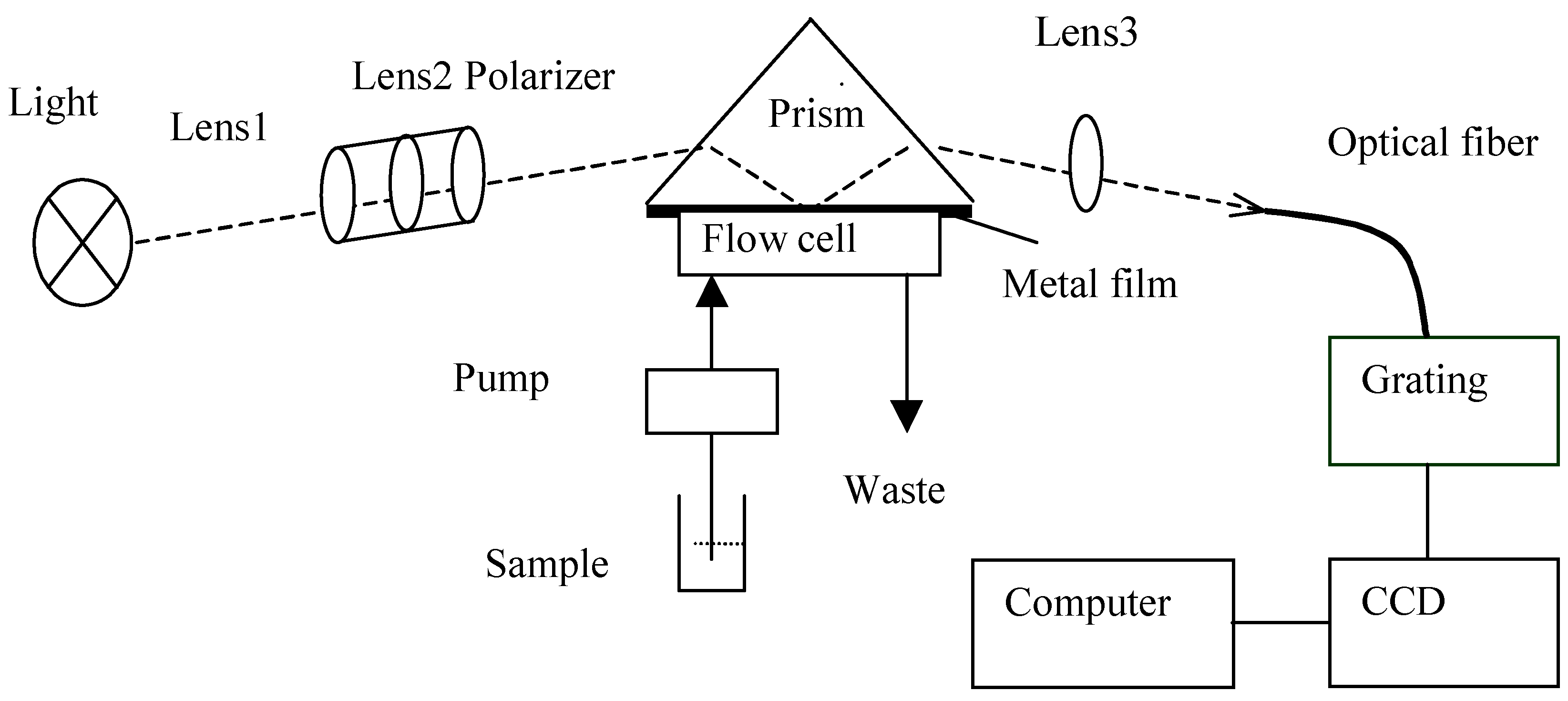
Self-assembled monolayers
Results and Discussion
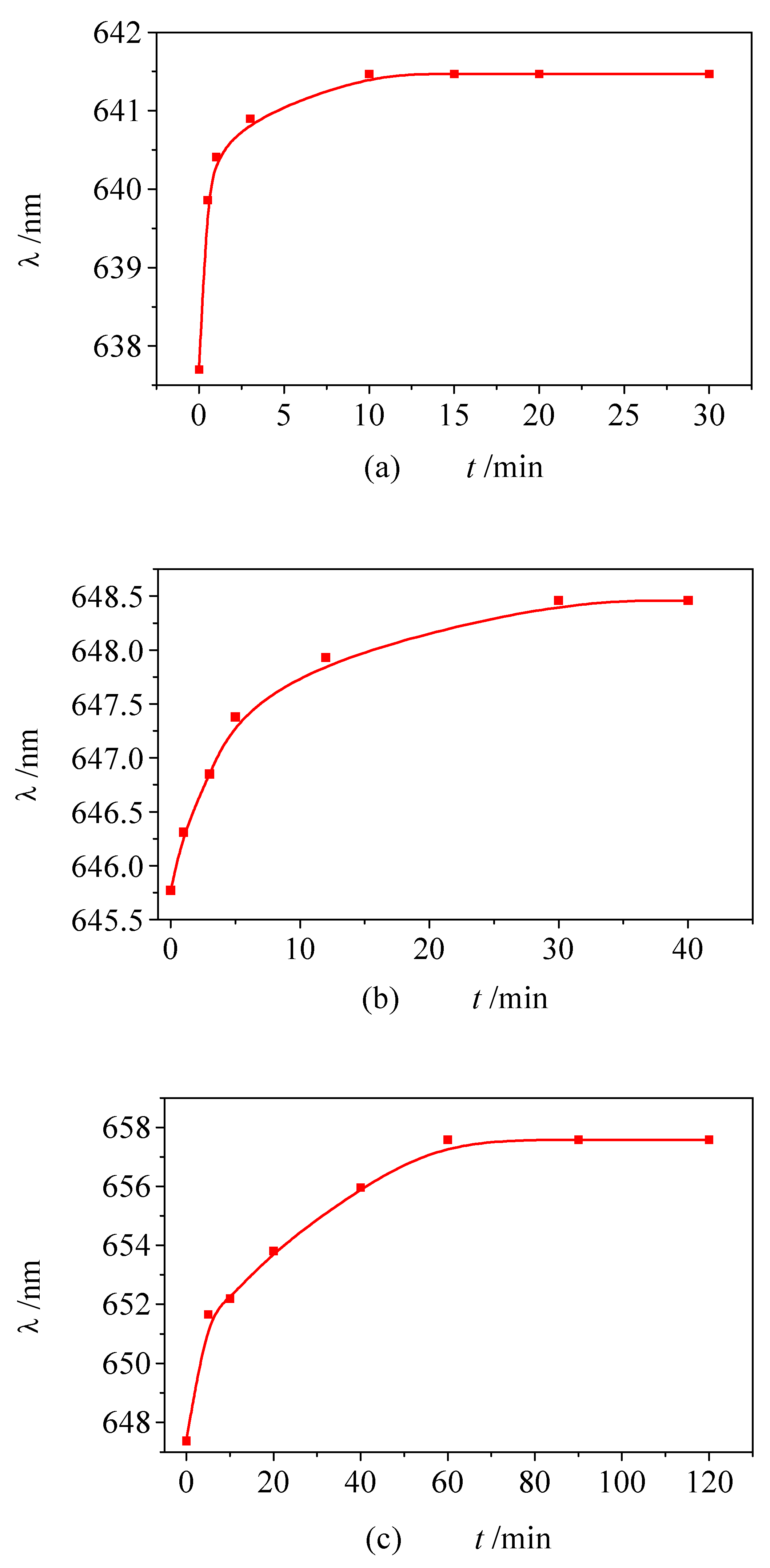
Monolayer formation
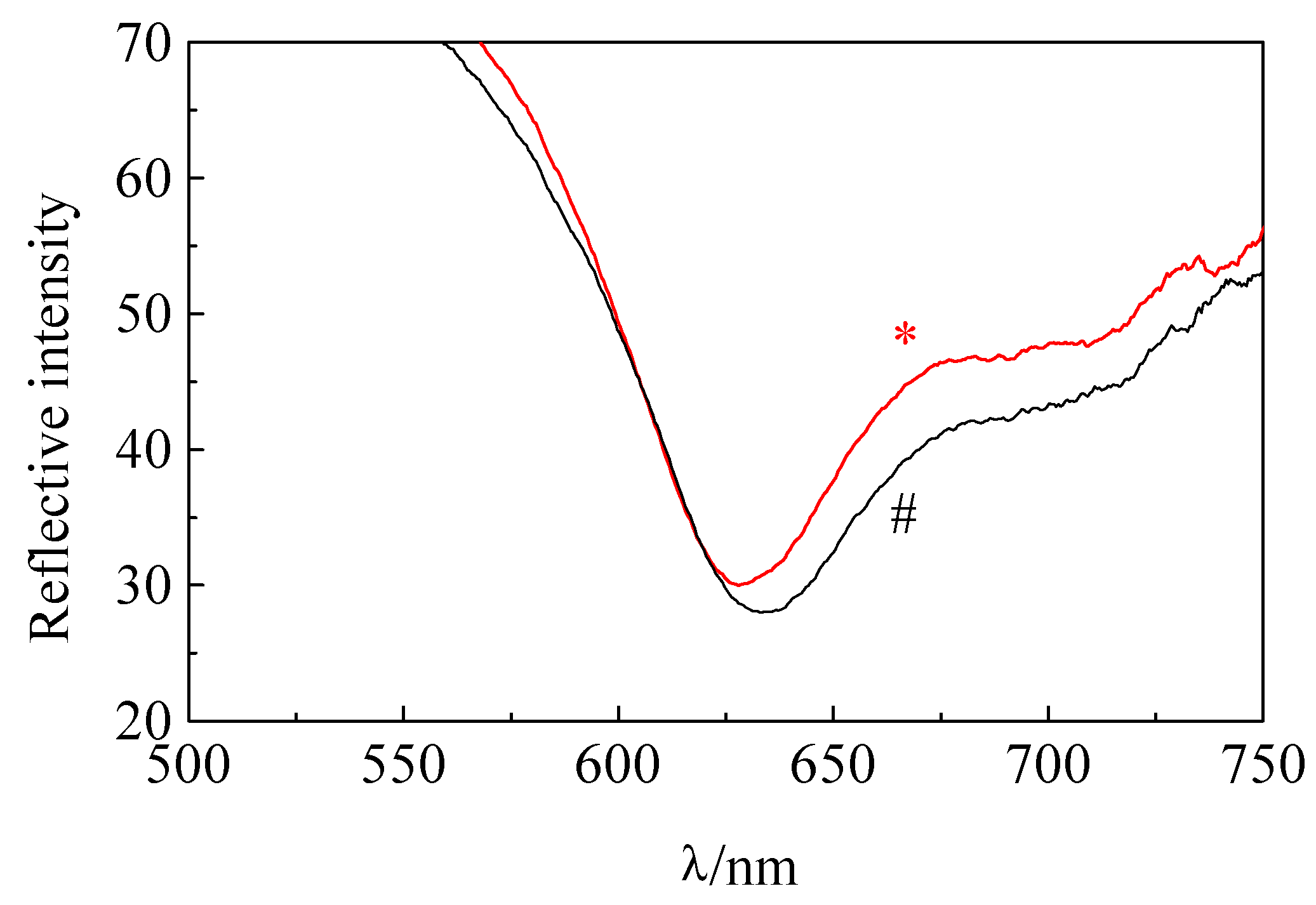
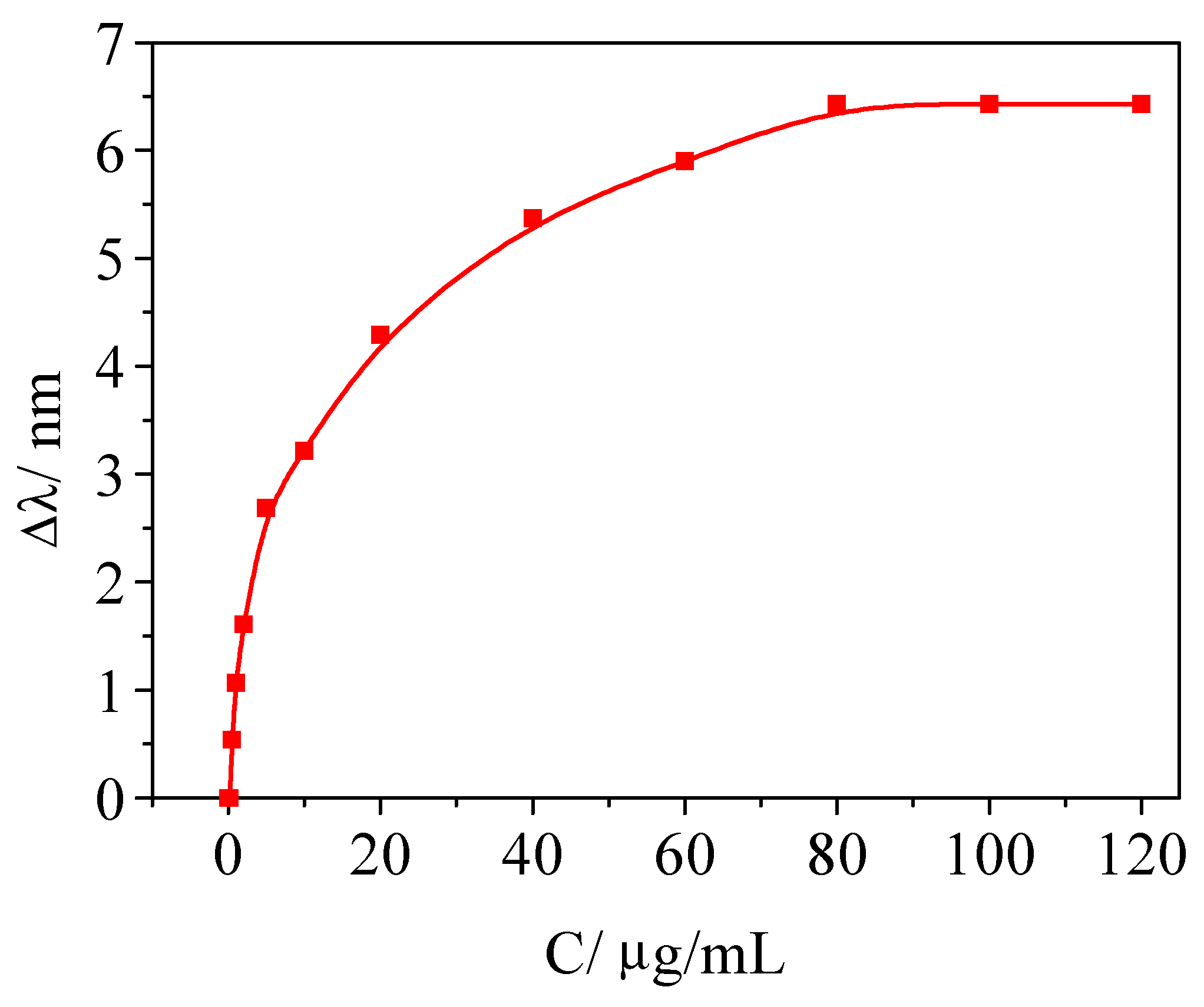
Determination of Bf
Selectivity
Regeneration

Conclusions
Acknowledgments
References
- Jansson, M.; Uhlenm, M.; Nilsson, B. Structural Changes in insulin-liked growth factor (IGF) I mutant proteins affecting binding kinetic rates to IGF binding protein l and IGF-I receptor. Science 1984, 123, 123–125. [Google Scholar]
- Mullett, W. M.; Lai, E. P. C.; Yeung, J. M. Surface plasmon resonance-based immunoassays. Methods 2000, 22, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Parsons, I. D.; Parson; Blk Mekhalfia, A.; Blackburn, G. M.; Stockley, P G. Probing the molecular mechanism of action of co-repressor in the E.coli methionine repressor-operator complex using surface plasmon resonance (SPR). Nucleic Acids Res. 1995, 23, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. M.; Uttamchandan, D. Optical chemical sensing employing surface plasmon resonance. Electronics Lett. 1988, 24, 1469–1470. [Google Scholar] [CrossRef]
- Jorgenson, R. C.; Yee, S. S.; Johnston, K. S.; Compton, B. J. A novel surface plasmon resonance based fiber optic sensor applied to biochemical sensing. SPIE. 1993, l886, 35–48. [Google Scholar]
- Jorgenson, R. C.; Yee, S. S. A fiber-optic chemical sensor based on surface plasmon resonance. Sens. Acutuators B 1993, 12, 213–220. [Google Scholar] [CrossRef]
- Jorgenson, K. S.; Karlson, S. R.; Jung, C.; Yee, S. S. New analytical technique for characterization of thin films using surface plasmon resonance. Mat.chem.phys. 1995, 42, 242–245. [Google Scholar]
- Englebienne, P. Use of colloidal gold surface plasmon resonance peak shift to infer affinity constants from the interactions between protein antigens and antibodies specific for single or multiple epitopes. Analyst 1998, 123, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. J.; Wang, Z.; Xu, H. Y.; Liang, F.; Zhang, H. Q.; Jin, Q. Studies on surface plasmon resonance sensor. Chem. J. Chinese Universities 1998, l9, 1214–1218. [Google Scholar]
- Zhao, X. J.; Mu, Y.; Wang, Z.; Zhang, H. Q.; Jin, Q. Studies on an immunosensor of albumin based surface plasmon resonance. Chem. J. Chinese Universities 1999, 20, 704–708. [Google Scholar]
- Mu, Y.; Zhang, H. Q.; Zhao, X. J.; Wang, Z.; Cao, Y B.; Jin, Q. An optical fibrin immunosensor based on surface plasmon resonance. Quim. Anal. 1999, 18, 277–282. [Google Scholar]
- Mu, Y.; Zhao, X. J.; Wang, Z.; Zhang, H. Q.; Jin, Q. The study on assembling process of IFN-γ DNA sensor by surface plasmon resonance. Acta Chimica Sinia. 2000, 58(5), 500–504. [Google Scholar]
- Zhao, X. J.; Wang, Z.; Mu, Y.; Zhang, H. Q.; Jin, Q. Simulating multiwavelength detection based on surface plasmon resonance technique. LRA. 2000, 12, 104–107. [Google Scholar]
- Quinn, J. G.; O'Neill, S.; Doyle, A.; McAtamney, C.; Diamond, D.; MacCraith, B. D.; O'Kennedy, R. Development and application of surface plasmon resonance-based biosensors for the detection of cell-ligand interactions. Anal. Biochem. 2000, 281, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Boussaad, S.; Wong, S.; Tao, N. J. High-sensitivity stark spectroscopy obtained by surface plasmon resonance measurement. Anal. Chem. 2000, 72, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Boussaad, S.; Pean, J.; Tao, N. J. High-resolution multiwavelength surface plasmon resonance spectroscopy for probing conformational and electronic changes in redox proteins. Anal. Chem. 2000, 72, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Wink, T.; Van Zuilen, S. J.; Bult, A.; Van Bennekom, W P. Liposome-mediated enhancement of the sensitivity in immunoassays of proteins and peptides in surface plasmon resonance spectrometer. Anal Chem. 1998, 70, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Silin, V; Weetall, H.; Vanderah, D. J. SPR studies of the nonspecific adsorption kinetics human IgG and BSA on gold surface modified by self assembled monolayers. J Cholloid Interface Sci. 1997, l85, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lyon, L. A.; Musick, M. D.; Natan, M. J. Colloidal Au-Enhanced surface plasmon resonance immunosensing. Anal. Chem. 1998, 70, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, J.; Isaacs, L.; Tien, J.; Whitesides, G. M. A strategy for the generation of surfaces presenting ligands for studies of binding based on an active ester as a common reactive intermediate: a surface plasmon resonance study. Anal. Chem. 1999, 71, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Calakos, N.; Bennett, K. E.; Peterson, K. E.; Scheller, R. H. Protein-protein interactions mediating the specificity of intracellular vesicular trafficking. Science 1994, 263, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Patti, J. M.; Boles, J. O.; Hssk, M. Identification and biochemical characterization of the ligand binding domain of the collagen adhesion from Staphylococcus aurous. Biochemistry 1993, 32, 11428–11435. [Google Scholar] [CrossRef] [PubMed]
- Hayer-Hedl, M. K.; Martin, J.; Hedl, F U. Asymmetrical interaction of GroEL and GroES in the ATPase cycle of assisted protein folding. Science 1995, 269, 836–841. [Google Scholar] [CrossRef]
- Mullett, W.; Lai, E. P. C.; Yeung, J. M. Immunoassay of fumonisins by a surface plasmon resonance biosensor. Anal. Biochem. 1998, 258, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Polymenis, M.; David Stollar, B. Domain interactions and antigen binding of recombinan anti-Z- DNA antibody variable domains f The role of heavy and light chains measured by surface plasmon resonance. J. Immunol. 1995, 154, 2198–2238. [Google Scholar] [PubMed]
- Andrea, A. D.; Rup, B. J.; Fisher, M. J.; Jones, S. Anti-Erythropoietin receptor (EPO-R) monoclonal antibodies inhibit erythropoietin binding and neutralize bioactivity. Blood 1993, 82, 46–52. [Google Scholar] [PubMed]
- Agrawal, S.; Chiistodoulou, C.; Gait, J. M. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotide. Nucleic Acids Res. 1986, 14, 6227–6245. [Google Scholar] [CrossRef] [PubMed]
- Chollet, A.; Dawashima, H. E. Biotin-labeled synthetic oligodeoxyribonucleotides: chemical synthesis and uses as hybridization probes. Nucleic Acids Res. 1985, 13, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; McGovern, M. E.; Thompson, M. Genosensor technology and the detection of interfacial nucleic acid chemistry. Anal. Chim. Acta. 1997, 346, 259–275. [Google Scholar] [CrossRef]
- Caruso, F; Rodda, E; Furlong, D. N. Quartz crystal microbalance study of DNA immobilization and hybridization for nucleic acid sensor development. Anal. Chem. 1997, 69, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Available from the author.
© 2001 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mu, Y.; Zhang, H.; Zhao, X.; Song, D.; Wang, Z.; Sun, J.; Li, M.; Jin, Q. An Optical Biosensor for Monitoring Antigen Recognition Based on Surface Plasmon Resonance Using Avidin-Biotin System. Sensors 2001, 1, 91-101. https://doi.org/10.3390/s10300091
Mu Y, Zhang H, Zhao X, Song D, Wang Z, Sun J, Li M, Jin Q. An Optical Biosensor for Monitoring Antigen Recognition Based on Surface Plasmon Resonance Using Avidin-Biotin System. Sensors. 2001; 1(3):91-101. https://doi.org/10.3390/s10300091
Chicago/Turabian StyleMu, Ying, Hanqi Zhang, Xiaojun Zhao, Daqian Song, Zhen Wang, Jing Sun, Minjing Li, and Qinhan Jin. 2001. "An Optical Biosensor for Monitoring Antigen Recognition Based on Surface Plasmon Resonance Using Avidin-Biotin System" Sensors 1, no. 3: 91-101. https://doi.org/10.3390/s10300091



