Known Predators of Crown-of-Thorns Starfish (Acanthaster spp.) and Their Role in Mitigating, If Not Preventing, Population Outbreaks
Abstract
:1. Introduction
1.1. The Predator Removal Hypothesis
1.2. Objectives of This Review
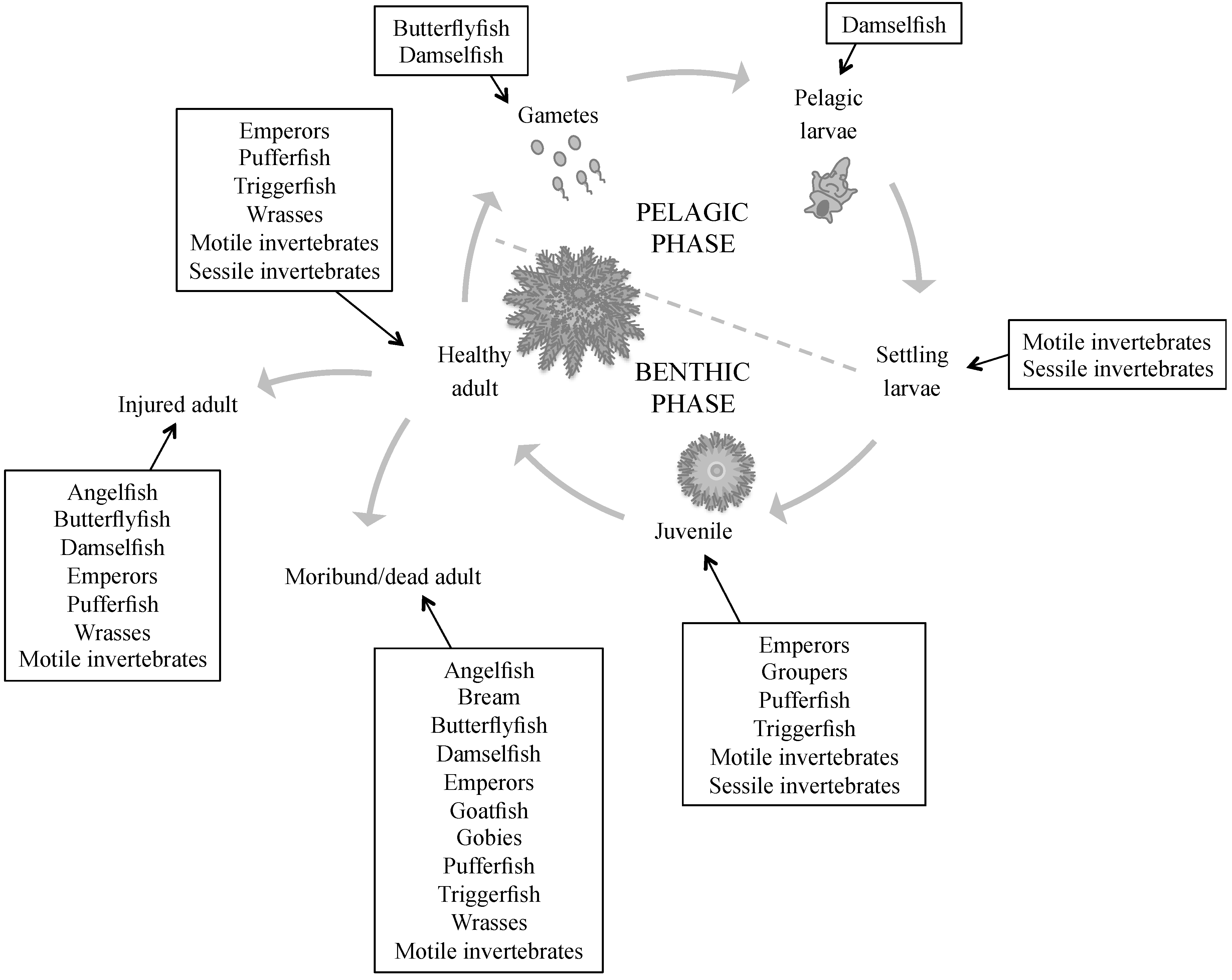
2. Known Predators of Crown-of-Thorns Starfish
2.1. Pre-Settlement Predation
2.2. Post-Settlement Predation
3. Rates of Predation on Crown-of-Thorns Starfish
3.1. Sub-Lethal Injuries
3.2. Population Modelling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Howden, M.E.H.; Lucas, J.S.; McDuff, M.; Salathe, R. Chemical defences of Acanthaster planci. In Crown-of-Thorns Starfish Seminar Proceedings; Australian Government Publishing Service: Canberra, Australia, 1975; pp. 67–79. [Google Scholar]
- Barnett, D.; Dean, P.W.; Hart, R.J.; Lucas, J.S.; Salathe, R.; Howden, M.E.H. Determination of contents of steroidal saponins in starfish tissues and study of their biosynthesis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 90, 141–145. [Google Scholar] [CrossRef]
- Shiomi, K.; Yamamoto, S.; Yamanaka, H.; Kikuchi, T. Purification and characterization of a lethal factor in venom from the crown-of-thorns starfish (Acanthaster planci). Toxicon 1988, 26, 1077–1083. [Google Scholar] [CrossRef]
- Lucas, J.; Hart, R.; Howden, M.; Salathe, R. Saponins in eggs and larvae of Acanthaster planci (L.) (Asteroidea) as chemical defences against planktivorous fish. J. Exp. Mar. Bio. Ecol. 1979, 40, 155–165. [Google Scholar] [CrossRef]
- Mackie, A.M.; Singh, H.T.; Fletcher, T.C. Studies on the cytolytic effects of seastar (Marthasterias glacialis) saponins and synthetic surfactants in the plaice Pleuronectes platessa. Mar. Biol. 1975, 29, 307–314. [Google Scholar] [CrossRef]
- Shiomi, K.; Yamamoto, S.; Yamanaka, H.; Kikuchi, T.; Konno, K. Liver damage by the crown-of-thorns starfish (Acanthaster planci) lethal factor. Toxicon 1990, 28, 469–475. [Google Scholar] [CrossRef]
- Shiomi, K.; Midorikawa, S.; Ishida, M.; Nagashima, Y.; Nagai, H. Plancitoxins, lethal factors from the crown-of-thorns starfish Acanthaster planci, are deoxyribonucleases II. Toxicon 2004, 44, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Early life histories of coral reef asteroids, with special reference to Acanthaster planci (L.). In Biology and Geology of Coral Reefs; Jones, O., Endean, R., Eds.; Academic Press: New York, NY, USA, 1973; pp. 369–387. [Google Scholar]
- Bertness, M.D.; Garrity, S.D.; Levings, S.C. Predation pressure and gastropod foraging: A tropical-temperate comparison. Evolution 1981, 35, 995–1007. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 133–200. [Google Scholar]
- Rivera-Posada, J.; Caballes, C.F.; Pratchett, M.S. Size-related variation in arm damage frequency in the crown-of-thorns sea star, Acanthaster planci. J. Coast. Life Med. 2014, 2, 187–195. [Google Scholar]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 343–366. [Google Scholar] [CrossRef]
- Pekár, S.; Liíznarová, E.; Řezáč, M. Suitability of woodlice prey for generalist and specialist spider predators. Ecol. Entomol. 2015, 41, 123–130. [Google Scholar] [CrossRef]
- Messmer, V.; Pratchett, M.; Chong-Seng, K. Variation in incidence and severity of injuries among crown-of-thorns starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef. Diversity 2016. under review. [Google Scholar]
- McCallum, H.I.; Endean, R.; Cameron, A.M. Sublethal damage to Acanthaster planci as an index of predation pressure. Mar. Ecol. Prog. Ser. 1989, 56, 29–36. [Google Scholar] [CrossRef]
- Birkeland, C.; Lucas, J.S. Acanthaster Planci: Major Management Problem of Coral Reefs; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Keesing, J.; Halford, A. Field measurement of survival rates of juvenile Acanthaster planci, techniques and preliminary results. Mar. Ecol. Prog. Ser. 1992, 85, 107–114. [Google Scholar] [CrossRef]
- Zann, L.; Brodie, J.; Berryman, C.; Naqasima, M. Recruitment, ecology, growth and behavior of juvenile Acanthaster planci (L.) (Echinodermata: Asteroidea). Bull. Mar. Sci. 1987, 41, 561–575. [Google Scholar]
- Cowan, Z.-L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Benthic predators influence microhabitat preferences and settlement success of crown-of-thorns starfish larvae (Acanthaster cf. solaris). Diversity 2016, 8. [Google Scholar] [CrossRef]
- Cowan, Z.-L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Predation on crown-of-thorns starfish larvae by damselfishes. Coral Reefs 2016, 35, 1253–1262. [Google Scholar] [CrossRef]
- Moran, P.J. The Acanthaster phenomenon. Oceanogr. Mar. Biol. 1986, 24, 379–480. [Google Scholar]
- Endean, R. Report on Investigations Made into Aspects of the Current Acanthaster planci (Crown of Thorns) Infestations of Certain Reefs of the Great Barrier Reef; Queensland Department of Primary Industries (Fisheries Branch): Brisbane, Australia, 1969.
- Estes, J.A.; Palmisano, J.F. Sea otters: Their role in structuring nearshore communities. Science 1974, 185, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Smith, N.S.; Palmisano, J.F. Sea otter predation and community organization in the western Aleutian Islands, Alaska. Ecology 1978, 59, 822–833. [Google Scholar] [CrossRef]
- Estes, J.A.; Duggins, D.O. Sea otters and kelp forests in Alaska: Generality and variation in a community ecological paradigm. Ecol. Monogr. 1995, 65, 75–100. [Google Scholar] [CrossRef]
- Ling, S.D.; Johnson, C.R.; Frusher, S.D.; Ridgway, K.R. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl. Acad. Sci. USA 2009, 106, 22341–22345. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Pearson, R.G.; Endean, R. A Preliminary Study of the Coral Predator Acanthaster planci (L.) (Asteroidea) on the Great Barrier Reef; Queensland Department of Harbours and Marine: Brisbane, Queensland, Australia, 1969; Volume 3, pp. 27–55.
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Brodie, J.; Fabricius, K.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Potts, D.C. Crown-of-thorns starfish—Man-induced pest or natural phenomenon? In The Ecology of Pests: Some Australian Case Histories; Kitching, R., Jones, R., Eds.; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1981; pp. 55–86. [Google Scholar]
- Endean, R. Population explosions of Acanthaster planci and associated destruction of hermatypic corals in the Indo-West Pacific region. In Biology and Geology of Coral Reefs; Jones, O.A., Endean, R., Eds.; Academic Press: New York, NY, USA, 1973; pp. 389–438. [Google Scholar]
- Ormond, R.; Bradbury, R.; Bainbridge, S.; Fabricius, K.; Keesing, J.; de Vantier, L.; Medlay, P.; Steven, A. Test of a model of regulation of crown-of-thorns starfish by fish predators. In Acanthaster and the Coral Reef: A Theoretical Perspective; Bradbury, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 189–207. [Google Scholar]
- Sweatman, H.P. Commercial fishes as predators of adult Acanthaster planci. In Proceedings of the 8th International Coral Reef Symposium, Panama City, Panama, 24–29 June 1996; Lessios, H.A., Macintyre, I.G., Eds.; Volume 1, pp. 617–620.
- Mendonça, V.M.; Al Jabri, M.M.; Al Ajmi, I.; Al Muharrami, M.; Al Areimi, M.; Al Aghbari, H.A. Persistent and expanding population outbreaks of the corallivorous starfish Acanthaster planci in the Northwestern Indian Ocean: Are they through overfishing in coral reefs, or a response to a changing environment? Zool. Stud. 2010, 49, 108–123. [Google Scholar]
- Dulvy, N.K.; Freckleton, R.P.; Polunin, N.V.C. Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol. Lett. 2004, 7, 410–416. [Google Scholar] [CrossRef]
- Sweatman, H. No-take reserves protect coral reefs from predatory starfish. Curr. Biol. 2008, 18, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J. Preliminary observations of the decomposition of crown-of-thorns starfish, Acanthaster planci (L.). Coral Reefs 1992, 11, 115–118. [Google Scholar] [CrossRef]
- Glynn, P.W. An amphinoid worm predator of the crown-of-thorns sea star and general predation on asteroids in eastern and western pacific coral reefs. Bull. Mar. Sci. 1984, 35, 54–71. [Google Scholar]
- McCallum, H.I. Effects of predation on organisms with pelagic larval stages: Models of metapopulations. In Proceedings of the 6th International Coral Reef Symposium, Townsville, Australia, 8–12 August 1988; Choat, J.H., Barnes, D., Borowitzka, M.A., Coll, J.C., Davies, P.J., Flood, P., Hatcher, B.G., Hopley, D., Hutchings, P.A., Kinsey, D., et al., Eds.; Volume 2, pp. 101–106.
- McCallum, H.I. Effects of predation on Acanthaster: Age-structure metapopulation models. In Acanthaster and the Coral Reef: A Theoretical Perspective; Bradbury, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 208–219. [Google Scholar]
- Morello, E.B.; Plagányi, É.E.; Babcock, R.C.; Sweatman, H.; Hillary, R.; Punt, A.E. Model to manage and reduce crown-of-thorns starfish outbreaks. Mar. Ecol. Prog. Ser. 2014, 512, 167–183. [Google Scholar] [CrossRef]
- Ormond, R.F.; Campbell, A.C. Formation and breakdown of Acanthaster planci aggregations in the Red Sea. In Proceedings of the 2nd International Coral Reef Symposium, Brisbane, Australia, 22 June–2 July 1973; Cameron, A.M., Cambell, B.M., Cribb, A.B., Endean, R., Jell, J.S., Jones, O.A., Mather, P., Talbot, F.H., Eds.; Volume 1, pp. 595–619.
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Uthicke, S.; Doyle, J.; Duggan, S.; Yasuda, N.; Mckinnon, A.D. Outbreak of coral-eating crown-of-thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Posada, J.; Pratchett, M.S.; Aguilar, C.; Grand, A.; Caballes, C.F. Bile salts and the single-shot lethal injection method for killing crown-of-thorns sea stars (Acanthaster planci). Ocean Coast. Manag. 2014, 102, 383–390. [Google Scholar] [CrossRef]
- Keesing, J.; Halford, A. Importance of postsettlement processes for the population dynamics of Acanthaster planci (L.). Mar. Freshw. Res. 1992, 43, 635–651. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Rivera-Posada, J. Controlling outbreaks of the coral-eating crown-of-thorns starfish using a single injection of common household vinegar. Coral Reefs 2016, 35, 223–228. [Google Scholar] [CrossRef]
- Lucas, J. Environmental influences on the early development of Acanthaster planci (L.). In Crown-of-Thorns Starfish Seminar Proceedings; Australian Government Publishing Service: Canberra, Australia, 1975; pp. 109–121. [Google Scholar]
- Sweatman, H.P.A. A field study of fish predation on juvenile crown-of-thorns starfish. Coral Reefs 1995, 14, 47–53. [Google Scholar] [CrossRef]
- Birdsey, R. Large Reef Fishes as Potential Predators of Acanthaster Planci: A Pilot Study by Alimentary Tract Analysis of Predatory Fishes from Reefs Subject to Acanthaster Feeding; Report to the Great Barrier Reef Marine Park Authority: Townsville, Australia, 1988; unpublished.
- Endean, R. Destruction and recovery of coral reef communities. In Biology and Geology of Coral Reefs; Jones, O., Endean, R., Eds.; Academic Press: New York, NY, USA, 1976; Volume 3, Biology 2; pp. 215–254. [Google Scholar]
- Ormond, R.; Campbell, A.; Head, S.; Moore, R.; Rainbow, P.; Saunders, A. Formation and breakdown of aggregations of the crown-of-thorns starfish, Acanthaster planci (L.). Nature 1973, 246, 167–169. [Google Scholar] [CrossRef]
- Owens, D. Acanthaster planci starfish in Fiji: Survey of incidence and biological studies. Fiji Agric. J. 1971, 33, 15–23. [Google Scholar]
- Chesher, R.H. Acanthaster planci: Impact on Pacific Coral Reefs; Final report to U.S. Department of the Interior; Westinghouse Research Laboratories: Pittsburgh, PA, USA, 1969; p. 151. [Google Scholar]
- Randall, J.E.; Head, S.M.; Sanders, A.P. L. Food habits of the giant humphead wrasse, Cheilinus undulatus (Labridae). Environ. Biol. Fishes 1978, 3, 235–238. [Google Scholar] [CrossRef]
- Alcala, A.C. The sponge crab Dromidiopsis dormia as a predator of the crown of thorns starfish. Silliman J. 1974, 21, 174–177. [Google Scholar]
- Wickler, W.; Seibt, U. Das Verhalten von Hymenocera picta Dana, einer Seesterne fressenden Garnele (Decapoda, Natantia, Gnathophyllidae). Z. Tierpsychol. 1970, 27, 352–368. [Google Scholar] [CrossRef]
- Glynn, P.W. Acanthaster population regulation by a shrimp and a worm. In Proceedings of the 4th International Coral Reef Symposium, Manilla, Philippines, 18–22 May 1981; Gomez, E.D., Birkeland, C.E., Buddemeier, R.W., Johannes, R.E., Marsh, J.A., Jr., Tsuda, R.T., Eds.; 1982; Volume 2, pp. 607–612. [Google Scholar]
- Brown, T.W. Starfish menaces coral reefs. Hemisphere 1970, 14, 31–36. [Google Scholar]
- Bos, A.R.; Mueller, B.; Gumanao, G.S. Feeding biology and symbiotic relationships of the corallimorpharian Paracorynactis hoplites (Anthozoa: Hexacorallia). Raffles Bull. Zool. 2011, 59, 245–250. [Google Scholar]
- Bos, A.R.; Gumanao, G.S.; Salac, F.N. A newly discovered predator of the crown-of-thorns starfish. Coral Reefs 2008, 27. [Google Scholar] [CrossRef]
- Ciarapica, G.; Passeri, L. An overview of the maldivian coral reefs in Felidu and North Male Atoll (Indian Ocean): Platform drowning by ecological crises. Facies 1993, 28, 33–65. [Google Scholar] [CrossRef]
- Babcock, R.C.; Milton, D.A.; Pratchett, M.S. Relationships between size and reproductive output in the crown-of-thorns starfish. Mar. Biol. 2016, 163, 1–7. [Google Scholar] [CrossRef]
- Babcock, R.C. Spawning behaviour of Acanthaster planci. Coral Reefs 1990, 9, 124. [Google Scholar] [CrossRef]
- Babcock, R.C.; Mundy, C.N. Reproductive biology, spawning and field fertilization rates of Acanthaster planci. Aust. J. Mar. Freshw. Res. 1992, 43, 525–534. [Google Scholar] [CrossRef]
- Bailey, K.M.; Houde, E.D. Predation on eggs and larvae of marine fishes and the recruitment problem. Adv. Mar. Biol. 1989, 25, 1–83. [Google Scholar]
- Fabricius, K.E.; Metzner, J. Scleractinian walls of mouths: Predation on coral larvae by corals. Coral Reefs 2004, 23, 245–248. [Google Scholar] [CrossRef]
- Cowan, Z.-L.; Ling, S.D.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Inter-specific variation in potential importance of planktivorous damselfishes as predators of Acanthaster sp. eggs. Coral Reefs 2016. under review. [Google Scholar]
- Keesing, J.K.; Wiedermeyer, W.L.; Okaji, K.; Halford, A.R.; Hall, K.C.; Cartwright, C.M. Mortality rates of juvenile starfish Acanthaster planci and Nardoa spp. measured on the Great Barrier Reef, Australia and in Okinawa, Japan. Oceanol. Acta 1996, 19, 441–448. [Google Scholar]
- Roegner, G.C.; Mann, R. Early recruitment and growth of the American oyster Crassostrea virginica (Bivalvia: Ostreidae) with respect to tidal zonation and season. Mar. Ecol. Prog. Ser. Oldend. 1995, 117, 91–101. [Google Scholar] [CrossRef]
- Almany, G.R.; Webster, M.S. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs 2006, 25, 19–22. [Google Scholar] [CrossRef]
- Chong-Seng, K.M.; Graham, N.A.J.; Pratchett, M.S. Bottlenecks to coral recovery in the Seychelles. Coral Reefs 2014, 33, 449–461. [Google Scholar] [CrossRef]
- Glynn, P.W. Interactions between Acanthaster and Hymenocera in the field and laboratory. In Proceedings: the 3rd International Coral Reef Symposium, Miami, Florida, USA; Taylor, D.L., Ed.; University of Miami: Miami, FL, USA, 1977; Volume 1, pp. 209–216. [Google Scholar]
- Wickler, W. Biology of Hymenocera picta Dana. Micronesica 1973, 9, 225–230. [Google Scholar]
- Ling, S.D.; Johnson, C.R. Marine reserves reduce risk of climate-driven phase shift by reinstating size-and habitat-specific trophic interactions. Ecol. Appl. 2012, 22, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.W. Individual recognition and phenotypic variability in Acanthaster planci (Echinodermata: Asteroidea). Coral Reefs 1982, 1, 89–94. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Muthiga, N.A. Patterns of predation on a sea urchin, Echinometra mathaei (de Blainville), on Kenyan coral reefs. J. Exp. Mar. Bio. Ecol. 1989, 126, 77–94. [Google Scholar] [CrossRef]
- Bonaviri, C.; Fernández, T.V.; Badalamenti, F.; Gianguzza, P.; Di Lorenzo, M.; Riggio, S. Fish versus starfish predation in controlling sea urchin populations in Mediterranean rocky shores. Mar. Ecol. Prog. Ser. 2009, 382, 129–138. [Google Scholar] [CrossRef]
- Potts, D. Crown-of-thorns starfish—Man-induced pest or natural phenomenon? In The Ecology of Pests: Some Australian Case Histories; Kitching, R.E., Jones, R.E., Eds.; CSIRO: Melbourne, Australia, 1982; pp. 55–86. [Google Scholar]
- Chesher, R.H. Destruction of Pacific corals by the sea star Acanthaster planci. Science 1969, 165, 280–283. [Google Scholar] [CrossRef] [PubMed]
- McCallum, H.I. Predator regulation of Acanthaster planci. J. Theor. Biol. 1987, 127, 207–220. [Google Scholar] [CrossRef]
- McCallum, H. Completing the circle: Stock-recruitment relationships and Acanthaster. Mar. Freshw. Res. 1992, 43, 653–662. [Google Scholar] [CrossRef]
- Ormond, R.F.G.; Campbell, A.C. Observations on Acanthaster planci and other coral reef echinoderms in the Sudanese Red Sea. In Symposia of the Zoological Society of London; Academic Press: London, UK, 1971; Volume 28, pp. 433–454. [Google Scholar]
- McClanahan, T.R. Kenyan coral reef-associated gastropod fauna: A comparison between protected and unprotected reefs. Mar. Ecol. Prog. Ser. 1989, 53, 11–20. [Google Scholar] [CrossRef]
- Endean, R. Acanthaster planci infestations of reefs of the Great Barrier Reef. In Proceedings: the 3rd International Coral Reef Symposium, Miami, Florida, USA; Taylor, D.L., Ed.; University of Miami: Miami, FL, USA, 1977; Volume 1, pp. 185–191. [Google Scholar]
- McClanahan, T.R.; Muthiga, N.A. Similar impacts of fishing and environmental stress on calcifying organisms in Indian Ocean coral reefs. Mar. Ecol. Prog. Ser. 2016, 560, 87–103. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Vasquez, J. The effect of sublethal predation on the biology of echinoderms. Oceanol. Acta 1996, 19, 431–440. [Google Scholar]
- Ling, S.D.; Johnson, C.R. Native spider crab causes high incidence of sub-lethal injury to the introduced seastar Asterias amurensis. In Echinoderms in a Changing World: Proceedings of the 13th International Echinoderm Conference, January 5–9 2009, University of Tasmania, Hobart Tasmania, Australia; CRC Press: New York, NY, USA, 2012; pp. 195–201. [Google Scholar]
- Rivera-Posada, J.A.; Pratchett, M.; Cano-Gómez, A.; Arango-Gómez, J.D.; Owens, L. Injection of Acanthaster planci with thiosulfate-citrate-bile-sucrose agar (TCBS). I. Disease induction. Dis. Aquat. Organ. 2011, 97, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Posada, J.; Owens, L.; Caballes, C.F.; Pratchett, M.S. The role of protein extracts in the induction of disease in Acanthaster planci. J. Exp. Mar. Bio. Ecol. 2012, 429, 1–6. [Google Scholar] [CrossRef]
- Caballes, C.F.; Schupp, P.J.; Pratchett, M.S.; Rivera-Posada, J.A. Interspecific transmission and recovery of TCBS-induced disease between Acanthaster planci and Linckia guildingi. Dis. Aquat. Organ. 2012, 100, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M. Arm loss and regeneration in Asteroidea (Echinodermata). Echinoderm Res. 1991, 1992, 39–52. [Google Scholar]
- Lawrence, J.M. Energetic costs of loss and regeneration of arms in stellate echinoderms. Integr. Comp. Biol. 2010, 50, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Messmer, V.; Pratchett, M.S.; Clark, T.D. Capacity for regeneration in crown-of-thorns starfish, Acanthaster planci. Coral Reefs 2013, 32, 461. [Google Scholar] [CrossRef]
- Sigl, R.; Steibl, S.; Laforsch, C. The role of vision for navigation in the crown-of-thorns seastar, Acanthaster planci. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Schoener, T.W. Inferring the properties of predation and other injury-producing agents from injury frequencies. Ecology 1979, 60, 1110–1115. [Google Scholar] [CrossRef]
- Marrs, J.; Wilkie, I.C.; Sköld, M.; Maclaren, W.M.; McKenzie, J.D. Size-related aspects of arm damage, tissue mechanics, and autotomy in the starfish Asterias rubens. Mar. Biol. 2000, 137, 59–70. [Google Scholar] [CrossRef]
- Ling, S.D.; Johnson, C.R.; Mundy, C.N.; Morris, A.; Ross, D.J. Hotspots of exotic free-spawning sex: Man-made environment facilitates success of an invasive seastar. J. Appl. Ecol. 2012, 49, 733–741. [Google Scholar] [CrossRef]
- Benzie, J.A.H.; Black, K.P.; Moran, P.J.; Dixon, P. Small-scale dispersion of eggs and sperm of the crown-of-thorns starfish (Acanthaster planci) in a shallow coral reef habitat. Biol. Bull. 1994, 186, 153–167. [Google Scholar] [CrossRef]
- Endean, R. Acanthaster planci on the Great Barrier Reef. In Proceedings of the 2nd International Coral Reef Symposium, Brisbane, Australia, 22 June–2 July 1973; Cameron, A.M., Cambell, B.M., Cribb, A.B., Endean, R., Jell, J.S., Jones, O.A., Mather, P., Talbot, F.H., Eds.; Volume 1, pp. 563–576.
- Hassell, M.P. The Dynamics of Arthropod Predator-Prey Systems; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Holling, C. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Entomol. Soc. Can. 1965, 97, 5–60. [Google Scholar] [CrossRef]
- Abrams, P.A. The effects of adaptive behavior on the Type-2 functional response. Ecology 1990, 71, 877–885. [Google Scholar] [CrossRef]
- Buckel, J.A.; Stoner, A.W. Functional response and switching behaviour of young-of-the-year piscivorus bluefish. J. Exp. Mar. Bio. Ecol. 2000, 245, 25–41. [Google Scholar] [CrossRef]
- Nilsson, P.A.; Ruxton, G.D. Temporally fluctuating prey and interfering predators: A positive feedback. Anim. Behav. 2004, 68, 159–165. [Google Scholar] [CrossRef]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Kaspari, M. Prey preparation and the determinants of handling time. Anim. Behav. 1990, 40, 118–126. [Google Scholar] [CrossRef]
- Baker, D.J.; Stillman, R.A.; Smith, B.M.; Bullock, J.M.; Norris, K.J. Vigilance and the functional response of granivorous foragers. Funct. Ecol. 2010, 24, 1281–1290. [Google Scholar] [CrossRef]
- Murdoch, W.W. Switching in general predators: Experiments on predator specificity and stability of prey populations. Ecol. Monogr. 1969, 39, 335–354. [Google Scholar] [CrossRef]
- Babcock, R.C.; Dambacher, J.M.; Morello, E.B.; Plagányi, É.E.; Hayes, K.R.; Sweatman, H.P.A.; Pratchett, M.S. Assessing different causes of crown-of-thorns starfish outbreaks and appropriate responses for management on the Great Barrier Reef. PLoS ONE 2016, 11, e0169048. [Google Scholar] [CrossRef] [PubMed]
- Redd, K.S.; Ling, S.D.; Frusher, S.D.; Jarman, S.; Johnson, C.R. Using molecular prey detection to quantify rock lobster predation on barrens-forming sea urchins. Mol. Ecol. 2014, 23, 3849–3869. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, L.D.; Babcock, R.C.; Hyndes, G.A. Movements of the western rock lobster (Panulirus cygnus) within shallow coastal waters using acoustic telemetry. Mar. Freshw. Res. 2008, 59, 603–613. [Google Scholar] [CrossRef]


| Predator | Sperm | Eggs | Larvae | Juvenile | Adult–Healthy | Adult–Injured | Adult–Moribund/Dead | Reference |
|---|---|---|---|---|---|---|---|---|
| Fishes | ||||||||
| Angelfish | ||||||||
| Holacanthus passer | L | [39] | ||||||
| Pomacanthus semicirculatus | F | [38] | ||||||
| Pomacanthus sexstriatus | F | [38] | ||||||
| Bream | ||||||||
| Scolopsis bilineatus | F | [46] | ||||||
| Butterflyfish | ||||||||
| Chaetodon aureofasciatus | F | [46] | ||||||
| Chaetodon auriga | L | FL | [11,38,46] | |||||
| Chaetodon auripes | F | [47] | ||||||
| Chaetodon citrinellus | L | [39] | ||||||
| Chaetodon plebeius | F | [46] | ||||||
| Chaetodon rafflesi | F | [46] | ||||||
| Chaetodon rainfordi | F | [46] | ||||||
| Chaetodon vagabundus | FL | [46,48] | ||||||
| Damselfish | ||||||||
| Abudefduf sexfasciatus | F | L | [20,22] | |||||
| Acanthochromis polyacanthus | L | L | [20,48] | |||||
| Amblyglyphidodon curacao | F | L | [20,28] | |||||
| Chromis atripectoralis | L | [20] | ||||||
| Chromis caerulea | L | F | [11,38] | |||||
| Chromis dimidiata | L | [49] | ||||||
| Chromis viridis | L | [20] | ||||||
| Chrysiptera rollandi | L | [20] | ||||||
| Dascyllus aruanus | L | [20] | ||||||
| Dascyllus reticulatus | L | [20] | ||||||
| Neoglyphidodon melas | F | [46] | ||||||
| Neoglyphidodon oxyodon | F | [46] | ||||||
| Neopomacentrus azysron | L | [20] | ||||||
| Pomacentrus amboinensis | L | [20] | ||||||
| Pomacentrus chrysurus | F | [46] | ||||||
| Pomacentrus moluccensis | L | L | FL | [11,20,38,46] | ||||
| Pomacentrus wardi | F | [46] | ||||||
| Stegastes acapulcoensis | F | [39] | ||||||
| Stegastes nigricans | F | [46] | ||||||
| Emperors | ||||||||
| Lethrinus atkinsoni | F | FL | [46,50] | |||||
| Lethrinus miniatus | F | [50,51] | ||||||
| Lethrinus nebulosus | G | F | [46,51] | |||||
| Goatfish | ||||||||
| Parupeneus multifasciatus | F | [46] | ||||||
| Gobies | ||||||||
| Cryptocentrus sp. | F | [38] | ||||||
| Groupers | ||||||||
| Epinephelus lanceolatus | FG | [52] | ||||||
| Pufferfish | ||||||||
| Arothron hispidus | F | FL | L | FL | [11,39,43,46,48,53] | |||
| Arothron manilensis | L | [46] | ||||||
| Arothron meleagris | F | [39] | ||||||
| Arothron nigropunctatus | F | [38] | ||||||
| Arothron stellatus | F | [47] | ||||||
| Triggerfish | ||||||||
| Balistapus undulatus | F | [46] | ||||||
| Balistoides viridescens | L | FL | L | [11,43,46,48,53] | ||||
| Pseudobalistes flavimarginatus | L | FGL | [11,43,54] | |||||
| Rhinecanthus aculeatus | L | [48] | ||||||
| Sufflamen verres | F | [39] | ||||||
| Wrasses | ||||||||
| Cheilinus diagrammus | F | [38] | ||||||
| Cheilinus fasciatus | F | [38] | ||||||
| Cheilinus undulatus | FG | [43,51,55,56] | ||||||
| Coris caudomacula | F | [46] | ||||||
| Halichoeres melanurus | L | [48] | ||||||
| Thalassoma hardwicke | F | [38] | ||||||
| Thalassoma lucasanum | FL | [11,39] | ||||||
| Thalassoma lunare | FL | [46,48] | ||||||
| Thalassoma nigrofasciatum | F | [46] | ||||||
| Motile invertebrates | ||||||||
| Acanthaster planci | F | [38] | ||||||
| Alpheus sp. | F | [38] | ||||||
| Bursa rubeta | I | I | [57] in [21] | |||||
| Cassis cornuta | L | [55] | ||||||
| Charonia tritonis | F | FL | F | [22,28,32,38] | ||||
| Cymatorium lotorium | I | [43] | ||||||
| Dardanus sp. | I | [43] | ||||||
| Diadema mexicanum | F | [39] | ||||||
| Dromidiopsis dormia | I | [57] in [21] | ||||||
| Hymenocera elegans/picta | L | F | F | [39,58,59] | ||||
| Murex sp. | L | [55] | ||||||
| Neaxius glyptocercus | I | I | [60] in [21] | |||||
| Panulirus penicillatus | L | [18] | ||||||
| Pherecardia striata | F | F | F | [39,59] | ||||
| Trapezia flavopunctata | L | [19] | ||||||
| Trapezia bidentata | L | [19] | ||||||
| Trapezia cymodoce | L | [19] | ||||||
| Trizopagurus magnificus | F | [39] | ||||||
| Xanthidae | L | [49] | ||||||
| Sessile invertebrates | ||||||||
| Paracorynactis hoplites | F | F | [61] | |||||
| Platygyra sp. | L | Cowan Pers. obs. | ||||||
| Pocillopora damicornis | L | [8] | ||||||
| Pseudocorynactis sp. | F | [62] | ||||||
| Stoichactis sp. | L | [55] | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cowan, Z.-L.; Pratchett, M.; Messmer, V.; Ling, S. Known Predators of Crown-of-Thorns Starfish (Acanthaster spp.) and Their Role in Mitigating, If Not Preventing, Population Outbreaks. Diversity 2017, 9, 7. https://doi.org/10.3390/d9010007
Cowan Z-L, Pratchett M, Messmer V, Ling S. Known Predators of Crown-of-Thorns Starfish (Acanthaster spp.) and Their Role in Mitigating, If Not Preventing, Population Outbreaks. Diversity. 2017; 9(1):7. https://doi.org/10.3390/d9010007
Chicago/Turabian StyleCowan, Zara-Louise, Morgan Pratchett, Vanessa Messmer, and Scott Ling. 2017. "Known Predators of Crown-of-Thorns Starfish (Acanthaster spp.) and Their Role in Mitigating, If Not Preventing, Population Outbreaks" Diversity 9, no. 1: 7. https://doi.org/10.3390/d9010007






