Environmental Tipping Points for Sperm Motility, Fertilization, and Embryonic Development in the Crown-of-Thorns Starfish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Maintenance of Animals for Experiments
2.2. Preparation of Experimental Seawater
2.2.1. Water-Soluble Egg Extracts
2.2.2. Temperature
2.2.3. Salinity
2.2.4. pH
2.3. Sperm Speed and Motility
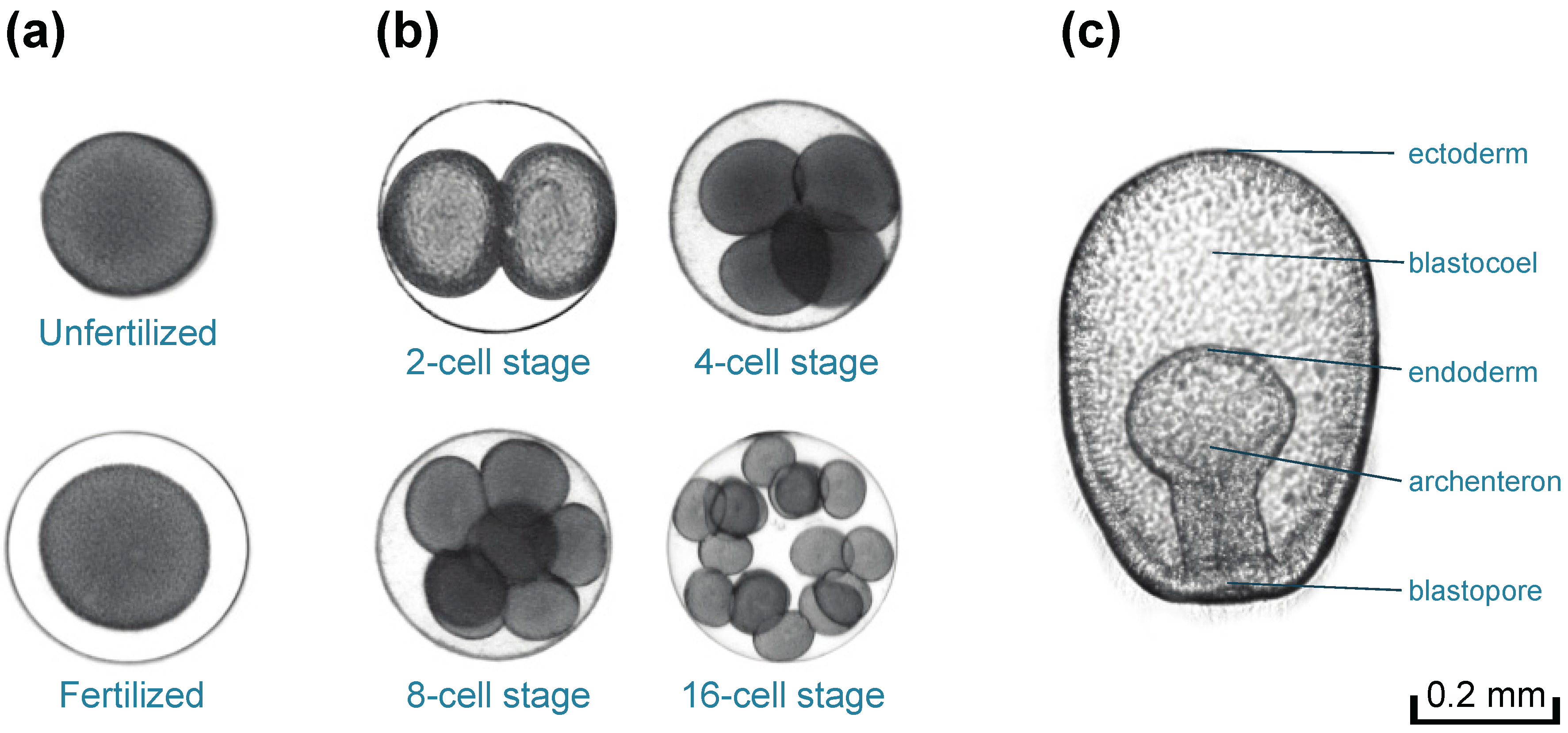
2.4. Bioassays for Fertilization and Embryonic Development
2.5. Statistical Analyses
3. Results
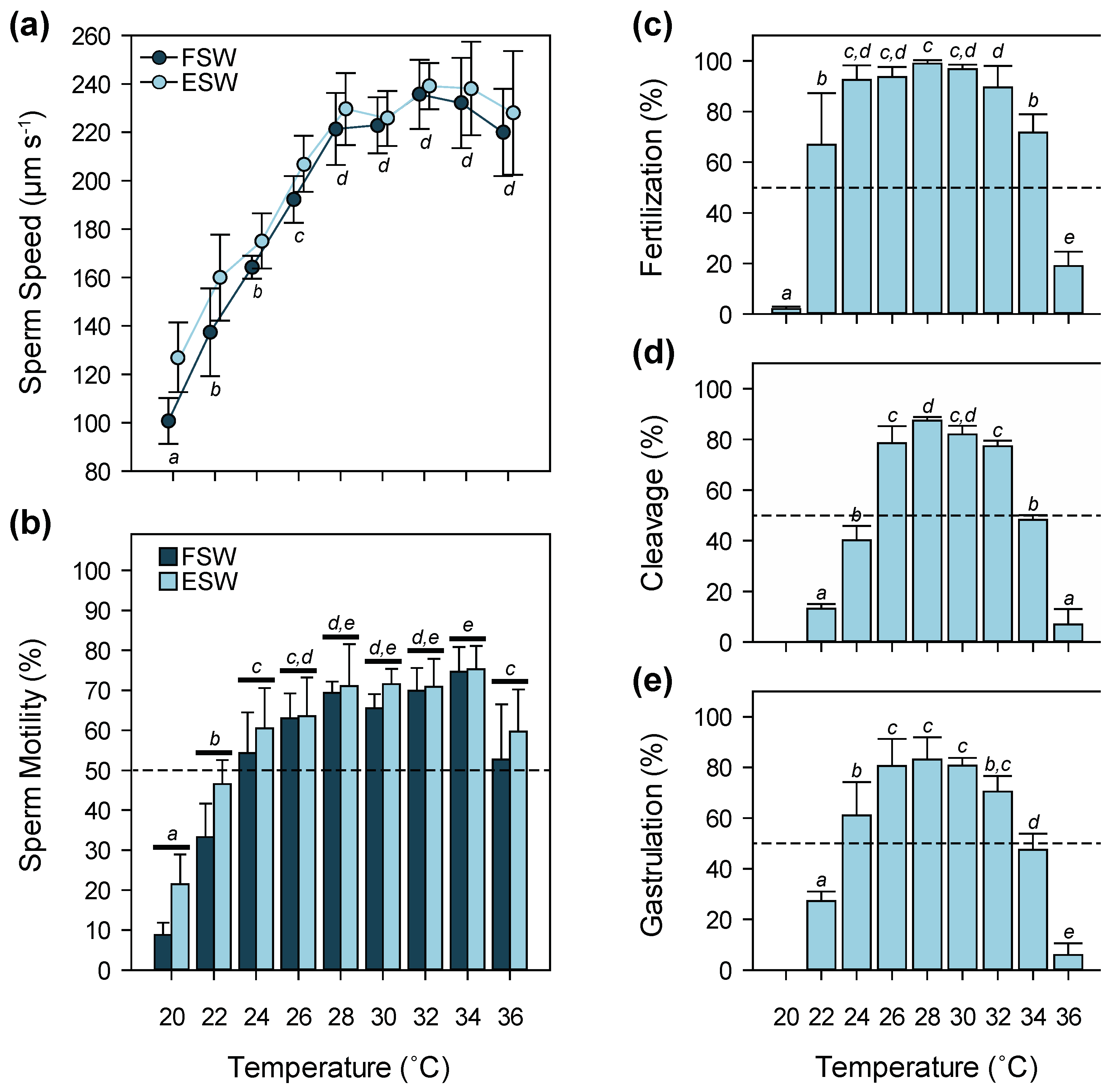
3.1. Temperature
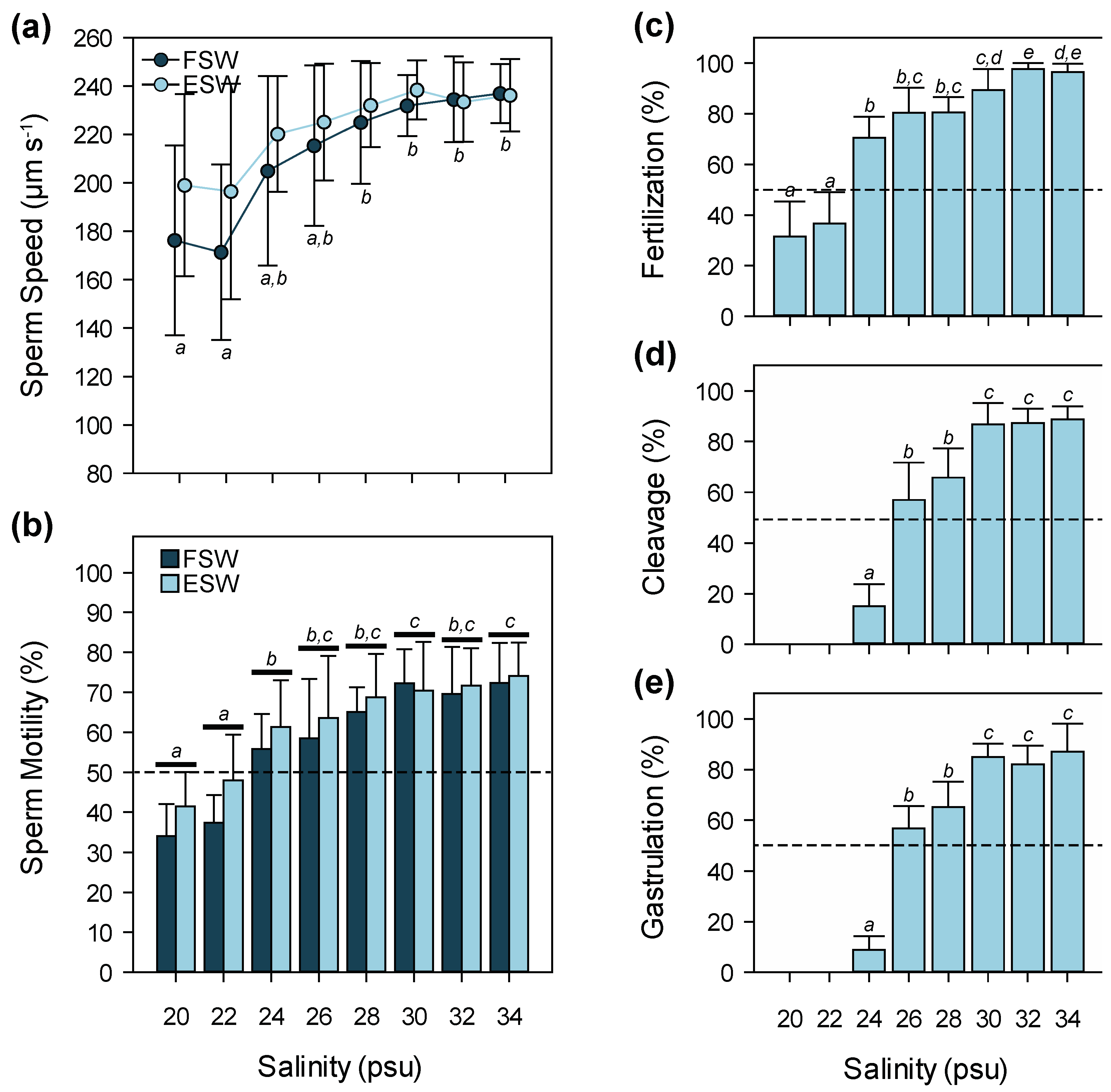
3.2. Salinity
3.3. pH
4. Discussion
4.1. Temperature
4.2. Salinity
4.3. pH
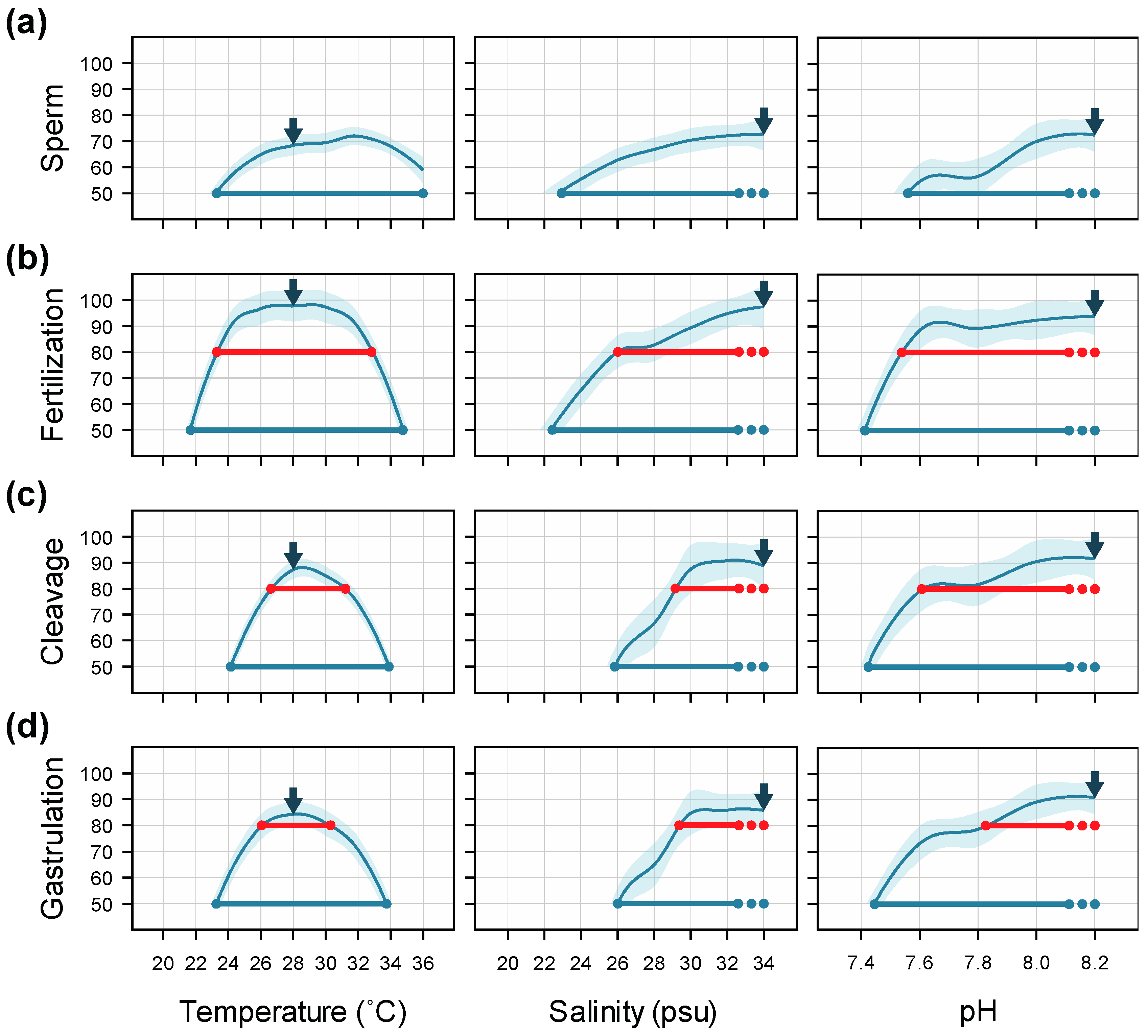
4.4. Interactive Effects and Implications for Subsequent Larval Development
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Source | DF | Statistic (F, χ2) | p |
|---|---|---|---|
| Temperature | |||
| Sperm Speed 1 | |||
| temperature | 8 | 85.96 | <0.0001 |
| egg extract | 1 | 13.16 | 0.0005 |
| temperature × egg extract | 8 | 0.76 | 0.6353 |
| Sperm Motility 2 | |||
| temperature | 8 | 1233.07 | <0.0001 |
| egg extract | 1 | 31.34 | 0.0008 |
| temperature × egg extract | 8 | 32.79 | 0.1612 |
| Fertilization 2 | 8 | 1316.20 | <0.0001 |
| Cleavage 2 | 7 | 521.09 | <0.0001 |
| Gastrulation 2 | 7 | 632.82 | <0.0001 |
| Salinity | |||
| Sperm Speed 1 | |||
| salinity | 7 | 5.83 | <0.0001 |
| egg extract | 1 | 2.93 | 0.0918 |
| salinity × egg extract | 7 | 0.31 | 0.9449 |
| Sperm Motility 2 | |||
| salinity | 7 | 525.43 | <0.0001 |
| egg extract | 1 | 16.42 | 0.0682 |
| salinity × egg extract | 7 | 9.62 | 0.9626 |
| Fertilization 2 | 7 | 597.86 | < 0.0001 |
| Cleavage 2 | 5 | 369.59 | < 0.0001 |
| Gastrulation 2 | 5 | 504.40 | < 0.0001 |
| pH | |||
| Sperm Speed 1 | |||
| pH | 4 | 28.57 | <0.0001 |
| egg extract | 1 | 3.85 | 0.0568 |
| pH × egg extract | 4 | 0.74 | 0.5706 |
| Sperm Motility 2 | |||
| pH | 4 | 669.24 | <0.0001 |
| egg extract | 1 | 38.11 | 0.0033 |
| pH × egg extract | 4 | 18.05 | 0.3943 |
| Fertilization 2 | 4 | 234.28 | <0.0001 |
| Cleavage 2 | 4 | 95.37 | <0.0001 |
| Gastrulation 2 | 4 | 213.24 | <0.0001 |
References
- De’ath, G.; Fabricius, K.E.; Sweatman, H.P.A.; Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.H.; Pratchett, M.S.; Hoey, A.S.; Herdiana, Y.; Campbell, S.J. Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs 2013, 32, 803–812. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. An Annu. Rev. 2014, 52, 133–200. [Google Scholar]
- Uthicke, S.; Schaffelke, B.; Byrne, M. A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol. Monogr. 2009, 79, 3–24. [Google Scholar] [CrossRef]
- Caballes, C.F.; Pratchett, M.S.; Kerr, A.M.; Rivera-Posada, J.A. The role of maternal nutrition on oocyte size and quality, with respect to early larval development in the coral-eating starfish, Acanthaster planci. PLoS ONE 2016, 11, e0158007. [Google Scholar] [CrossRef] [PubMed]
- Babcock, R.C.; Milton, D.A.; Pratchett, M.S. Relationships between size and reproductive output in the crown-of-thorns starfish. Mar. Biol. 2016, 163, 234. [Google Scholar] [CrossRef]
- Caballes, C.F.; Pratchett, M.S. Reproductive biology and early life history of the crown-of-thorns starfish. In Echinoderms: Ecology, Habitats and Reproductive Biology; Whitmore, E., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 101–146. [Google Scholar]
- Hoey, J.; Campbell, M.; Hewitt, C.; Gould, B.; Bird, R. Acanthaster planci invasions: Applying biosecurity practices to manage a native boom and bust coral pest in Australia. Manag. Biol. Invasions 2016, 7, 213–220. [Google Scholar] [CrossRef]
- Levitan, D.R. The ecology of fertilization in free-spawning invertebrates. In Ecology of Marine Invertebrate Larvae; McEdward, L.R., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 123–156. [Google Scholar]
- Conand, C. Distribution, reproductive cycle and morphometric relationships of Acanthaster planci (Echinodermata: Asteroidea) in New Caledonia, western tropical Pacific. In Proceedings of the 5th International Echinoderm Conference, Galway, Ireland, 24–29 September 1985; pp. 499–506.
- Yamaguchi, M. Recruitment of coral reef asteroids, with emphasis on Acanthaster planci (L.). Micronesica 1973, 9, 207–212. [Google Scholar]
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Hock, K.; Wolff, N.H.; Condie, S.A.; Anthony, K.R.N.; Mumby, P.J. Connectivity networks reveal the risks of crown-of-thorns starfish outbreaks on the Great Barrier Reef. J. Appl. Ecol. 2014, 51, 1188–1196. [Google Scholar] [CrossRef]
- Cowan, Z.L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Predation on crown-of-thorns starfish larvae by damselfishes. Coral Reefs 2016, 35, 1253–1262. [Google Scholar] [CrossRef]
- Uthicke, S.; Pecorino, D.; Albright, R.; Negri, A.P.; Cantin, N.; Liddy, M.; Dworjanyn, S.A.; Kamya, P.Z.; Byrne, M.; Lamare, M.D. Impacts of ocean acidification on early life-history stages and settlement of the coral-eating sea star Acanthaster planci. PLoS ONE 2013, 8, e82938. [Google Scholar] [CrossRef] [PubMed]
- Havenhand, J.N.; Buttler, F.R.; Thorndyke, M.C.; Williamson, J.E. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 2008, 18, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Havenhand, J.N.; Gillings, M.R.; Williamson, J.E. Individual variability in reproductive success determines winners and losers under ocean acidification: A case study with sea urchins. PLoS ONE 2012, 7, e53118. [Google Scholar] [CrossRef] [PubMed]
- Denny, M.W.; Shibata, M.F. Consequences of surf-zone turbulence for settlement and external fertilization. Am. Nat. 1989, 134, 859–889. [Google Scholar] [CrossRef]
- Levitan, D.R.; Sewell, M.A.; Chia, F.-S. How distribution and abundance influence fertilization success in the sea urchin Strongylocentotus franciscanus. Ecology 1992, 73, 248–254. [Google Scholar] [CrossRef]
- Babcock, R.C.; Mundy, C.N.; Whitehead, D. Sperm diffusion models and in situ confirmation of long-distance fertilization in the free-spawning asteroid Acanthaster planci. Biol. Bull. 1994, 186, 17–28. [Google Scholar] [CrossRef]
- Babcock, R.C.; Mundy, C.N. Reproductive biology, spawning and field fertilization rates of Acanthaster planci. Mar. Freshw. Res. 1992, 43, 525–534. [Google Scholar] [CrossRef]
- Benzie, J.A.H.; Dixon, P. The effects of sperm concentration, sperm: Egg ratio, and gamete age on fertilization success in crown-of-thorns starfish (Acanthaster planci) in the laboratory. Biol. Bull. 1994, 186, 139–152. [Google Scholar] [CrossRef]
- Byrne, M. Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 2012, 76, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Przeslawski, R.; Byrne, M.; Mellin, C. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Chang. Biol. 2015, 21, 2122–2140. [Google Scholar] [CrossRef] [PubMed]
- Helmuth, B.; Mieszkowska, N.; Moore, P.; Hawkins, S.J.; Hawkins, S.J. Living on the edge of two changing worlds: Forecasting the responses of rocky intertidal ecosystems to climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Pearse, J.S. Temperature, food availability, and the development of marine invertebrate larvae. Am. Zool. 1995, 35, 415–425. [Google Scholar] [CrossRef]
- Przeslawski, R.; Ahyong, S.; Byrne, M.; Wörheide, G.; Hutchings, P.A. Beyond corals and fish: The effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob. Chang. Biol. 2008, 14, 2773–2795. [Google Scholar] [CrossRef]
- Roberts, D.E.; Davis, A.R.; Cummins, S.P. Experimental manipulation of shade, silt, nutrients and salinity on the temperate reef sponge Cymbastela concentrica. Mar. Ecol. Prog. Ser. 2006, 307, 143–154. [Google Scholar] [CrossRef]
- Greenwood, P.J.; Bennett, T. Some effects of temperature-salinity combinations on the early development of the sea urchin Parechinus angulosus (Leske). Fertilization. J. Exp. Mar. Biol. Ecol. 1981, 51, 119–131. [Google Scholar] [CrossRef]
- Rupp, J.H. Effects of temperature on fertilization and early cleavage of some tropical echinoderms, with emphasis on Echinometra mathaei. Mar. Biol. 1973, 23, 183–189. [Google Scholar] [CrossRef]
- Kashenko, S.D. Responses of embryos and larvae of the starfish Asterias amurensis to changes in temperature and salinity. Russ. J. Mar. Biol. 2005, 31, 294–302. [Google Scholar] [CrossRef]
- Dupont, S.; Ortega-Martínez, O.; Thorndyke, M.C. Impact of near-future ocean acidification on echinoderms. Ecotoxicology 2010, 19, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.G.; Babcock, R.C. Temperature and the larval ecology of the crown-of-thorns starfish, Acanthaster planci. Biol. Bull. 1994, 187, 304–308. [Google Scholar] [CrossRef]
- Mita, M.; Nakamura, M. Energy metabolism of sea urchin spermatozoa: An approach based on echinoid phylogeny. Zool. Sci. 1998, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Ikegami, S.; Kanatani, H.; Mohri, H. Regulation of sperm motility in starfish. I. Initiation of movement. Dev. Growth Differ. 1982, 24, 419–428. [Google Scholar] [CrossRef]
- Lu, X.Y.; Wu, R.S.S. Ultraviolet damages sperm mitochondrial function and membrane integrity in the sea urchin Anthocidaris crassispina. Ecotoxicol. Environ. Saf. 2005, 61, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Wu, R.S.S. UV induces reactive oxygen species, damages sperm, and impairs fertilisation in the sea urchin Anthocidaris crassispina. Mar. Biol. 2005, 148, 51–57. [Google Scholar] [CrossRef]
- Kupriyanova, E.; Havenhand, J.N. Effects of temperature on sperm swimming behaviour, respiration and fertilization success in the serpulid polychaete, Galeolaria caespitosa (Annelida: Serpulidae). Invertebr. Reprod. Dev. 2005, 48, 7–17. [Google Scholar] [CrossRef]
- Dinnel, P.A.; Link, J.M.; Stober, Q.J. Improved methodology for a sea urchin sperm cell bioassay for marine waters. Arch. Environ. Contam. Toxicol. 1987, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L. Demonstration of sperm chemotaxis in Echinodermata: Asteroidea, Holothuroidea, Ophiuroidea. J. Exp. Zool. 1985, 234, 383–414. [Google Scholar]
- Cook, S.P.; Brokaw, C.J.; Muller, C.H.; Babcock, D.F. Sperm chemotaxis: Egg peptides control cytosolic calcium to regulate flagellar responses. Dev. Biol. 1994, 165, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, T.; Chiba, K.; Miki, W.; Hoshi, M. Structure and function of asterosaps, sperm-activating peptides from the jelly coat of starfish eggs. Zygote 1996, 4, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Bolton, T.F.; Havenhand, J.N. Chemical mediation of sperm activity and longevity in the solitary ascidians Ciona intestinalis and Ascidiella aspersa. Biol. Bull. 1996, 190, 329–335. [Google Scholar] [CrossRef]
- Litvak, M.K.; Trippel, E.A. Sperm motility patterns of Atlantic cod (Gadus morhua) in relation to salinity: Effects of ovarian fluid and egg presence. Can. J. Fish. Aquat. Sci. 1998, 55, 1871–1877. [Google Scholar] [CrossRef]
- Kupriyanova, E.; Havenhand, J.N. Variation in sperm swimming behaviour and its effect on fertilization success in the serpulid polychaete Galeolaria caespitosa. Invertebr. Reprod. Dev. 2002, 41, 21–26. [Google Scholar] [CrossRef]
- Byrne, M.; Ho, M.A.; Selvakumaraswamy, P.; Nguyen, H.D.; Dworjanyn, S.A.; Davis, A.R. Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc. R. Soc. B Biol. Sci. 2009, 276, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Pechenik, J.A. Understanding the effects of low salinity on fertilization success and early development in the sand dollar Echinarachnius parma. Biol. Bull. 2010, 218, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Byrne, M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential for persistence in a changing ocean. Oceanogr. Mar. Biol. Annu. Rev. 2011, 49, 1–42. [Google Scholar]
- Wilson-Leedy, J.G.; Ingermann, R.L. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 2007, 67, 661–672. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 31 May 2016).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biometrical J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.J. The R Book; John Wiley & Sons Ltd.: Sussex, UK, 2013. [Google Scholar]
- Cheney, D.P. Spawning and aggregation of Acanthaster planci in Micronesia. In Proceedings of the Second International Coral Reef Symposium; Great Barrier Reef Committee: Brisbane, Australia, 1974; Volume 1, pp. 591–594. [Google Scholar]
- Mita, M.; Hino, A.; Yasumasu, I. Effect of temperature on interaction between eggs and spermatozoa of sea urchin. Biol. Bull. 1984, 166, 68–77. [Google Scholar] [CrossRef]
- Yamada, K.; Mihashi, K. Temperature-independent period immediately after fertilization in sea urchin eggs. Biol. Bull. 1998, 195, 107–111. [Google Scholar] [CrossRef]
- Hamdoun, A.; Epel, D. Embryo stability and vulnerability in an always changing world. Proc. Natl. Acad. Sci. USA 2007, 104, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Hagström, B.E.; Hagström, B. The effect of decreased and increased temperatures on fertilization. Exp. Cell Res. 1959, 16, 174–183. [Google Scholar] [CrossRef]
- Andronikov, V.B. Heat resistance of gametes of marine invertebrates in relation to temperature conditions under which the species exist. Mar. Biol. 1975, 30. [Google Scholar] [CrossRef]
- Farmanfarmaian, A.; Giese, A.C. Thermal tolerance and acclimation in the western purple sea urchin, Strongylocentrotus purpuratus. Physiol. Zool. 1963, 36, 237–243. [Google Scholar] [CrossRef]
- Sparks, K.M.; Foo, S.A.; Uthicke, S.; Byrne, M.; Lamare, M.D. Paternal identity influences response of Acanthaster planci embryos to ocean acidification and warming. Coral Reefs 2017, 36, 325–338. [Google Scholar] [CrossRef]
- Habe, T.; Sawamoto, S.; Ueno, S.; Kosaka, M.; Ogura, M. Studies on the Conservation and Management of Coral Reefs and the Control of Acanthaster Planci Juveniles; Report of Grant-in-Aid for Scientific Research; Ministry of Education, Science and Culture: Tokyo, Japan, 1989; pp. 158–186. (In Japanese) [Google Scholar]
- Lamare, M.D.; Pecorino, D.; Hardy, N.; Liddy, M.; Byrne, M.; Uthicke, S. The thermal tolerance of crown-of-thorns (Acanthaster planci) embryos and bipinnaria larvae: Implications for spatial and temporal variation in adult populations. Coral Reefs 2014, 33, 207–219. [Google Scholar] [CrossRef]
- Johnson, L.G.; Chenoweth, J.E.; Bingham, B.L. Population differences and thermal acclimation in temperature responses of developing sea urchin embryos. Proc. S. D. Acad. Sci. 1990, 69, 99–108. [Google Scholar]
- Kashenko, S.D. The combined effect of temperature and salinity on development of the sea star Asterina pectinifera. Russ. J. Mar. Biol. 2006, 32, 37–44. [Google Scholar] [CrossRef]
- Kashenko, S.D. Adaptive responses of embryos and larvae of the heart-shaped sea urchin Echinocardium cordatum to temperature and salinity changes. Russ. J. Mar. Biol. 2007, 33, 381–390. [Google Scholar] [CrossRef]
- Kashenko, S.D. Combined effect of temperature and salinity on the development of the holothurian Eupentacta fraudatrix. Russ. J. Mar. Biol. 2000, 26, 188–193. [Google Scholar] [CrossRef]
- Roller, R.A.; Stickle, W.B. Effects of temperature and salinity acclimation of adults on larval survival, physiology, and early development of Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Biol. 1993, 116, 583–591. [Google Scholar] [CrossRef]
- Griffin, F.J.; Pillai, M.C.; Vines, C.A.; Kääriä, J.; Hibbard-Robbins, T.; Yanagimachi, R.; Cherr, G.N. Effects of salinity on sperm motility, fertilization, and development in the Pacific herring, Clupea pallasi. Biol. Bull. 1998, 194, 25–35. [Google Scholar] [CrossRef]
- Elofsson, H.; Van Look, K.; Borg, B.; Mayer, I. Influence of salinity and ovarian fluid on sperm motility in the fifteen-spined stickleback. J. Fish Biol. 2003, 63, 1429–1438. [Google Scholar] [CrossRef]
- Devlin, M.J.; DeBose, J.L.; Ajani, P.; Teixeira da Silva, E.; Petus, C.; Brodie, J.E. Phytoplankton in the Great Barrier Reef: Microscopy analysis of community structure in high flow events. In Report to the National Environmental Research Program; Reef and Rainforest Research Centre Limited: Cairns, Australia, 2013; p. 68. [Google Scholar]
- Johnson, C.H.; Clapper, D.L.; Winkler, M.M.; Lee, H.C.; Epel, D. A volatile inhibitor immobilizes sea urchin sperm in semen by depressing the intracellular pH. Dev. Biol. 1983, 98, 493–501. [Google Scholar] [CrossRef]
- Kamya, P.Z.; Dworjanyn, S.A.; Hardy, N.; Mos, B.; Uthicke, S.; Byrne, M. Larvae of the coral eating crown-of-thorns starfish, Acanthaster planci in a warmer-high CO2 ocean. Glob. Chang. Biol. 2014, 20, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Chia, F.-S.; Bickell, L.R. Echinodermata. In Reproductive Biology of Invertebrates, Volume 2; Adiyodi, K.G., Adiyodi, R.G., Eds.; New York, NY, USA, 1983; pp. 545–620. [Google Scholar]
- Levitan, D.R. Sperm velocity and longevity trade off each other and infuence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. B Biol. Sci. 2000, 267, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Vihtakari, M.; Havenhand, J.N.; Renaud, P.E.; Hendriks, I.E. Variable individual- and population-level responses to ocean acidification. Front. Mar. Sci. 2016, 3, 51. [Google Scholar] [CrossRef]
- Havenhand, J.N.; Schlegel, P. Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences 2009, 6, 3009–3015. [Google Scholar] [CrossRef]
- Schlegel, P.; Havenhand, J.N.; Obadia, N.; Williamson, J.E. Sperm swimming in the polychaete Galeolaria caespitosa shows substantial inter-individual variability in response to future ocean acidification. Mar. Pollut. Bull. 2014, 78, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.A. Preliminary observations on the rearing and development of Acanthaster planci (L.) (Asteroidea) larvae. Fish. Notes Queensl. Dep. Harb. Mar. 1969, 3, 69–79. [Google Scholar]
- Henderson, J.A.; Lucas, J.S. Larval development and metamorphosis of Acanthaster planci (Asteroidea). Nature 1971, 232, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval starvation to satiation: Influence of nutrient regime on the success of Acanthaster planci. PLoS ONE 2015, 10, e0122010. [Google Scholar] [CrossRef] [PubMed]
- Uthicke, S.; Logan, M.; Liddy, M.; Francis, D.S.; Hardy, N.; Lamare, M.D. Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci. Rep. 2015, 5, 8402. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.J.; Stewart, P.; Rivera-Posada, J.A. De novo assembly of the transcriptome of Acanthaster planci testes. Mol. Ecol. Resour. 2015, 15, 953–966. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballes, C.F.; Pratchett, M.S.; Raymundo, M.L.; Rivera-Posada, J.A. Environmental Tipping Points for Sperm Motility, Fertilization, and Embryonic Development in the Crown-of-Thorns Starfish. Diversity 2017, 9, 10. https://doi.org/10.3390/d9010010
Caballes CF, Pratchett MS, Raymundo ML, Rivera-Posada JA. Environmental Tipping Points for Sperm Motility, Fertilization, and Embryonic Development in the Crown-of-Thorns Starfish. Diversity. 2017; 9(1):10. https://doi.org/10.3390/d9010010
Chicago/Turabian StyleCaballes, Ciemon Frank, Morgan S. Pratchett, Maia L. Raymundo, and Jairo A. Rivera-Posada. 2017. "Environmental Tipping Points for Sperm Motility, Fertilization, and Embryonic Development in the Crown-of-Thorns Starfish" Diversity 9, no. 1: 10. https://doi.org/10.3390/d9010010






