Conservation Genetics of Crested Newt Species Triturus cristatus and T. carnifex within a Contact Zone in Central Europe: Impact of Interspecific Introgression and Gene Flow
Abstract
:1. Introduction
2. Methods
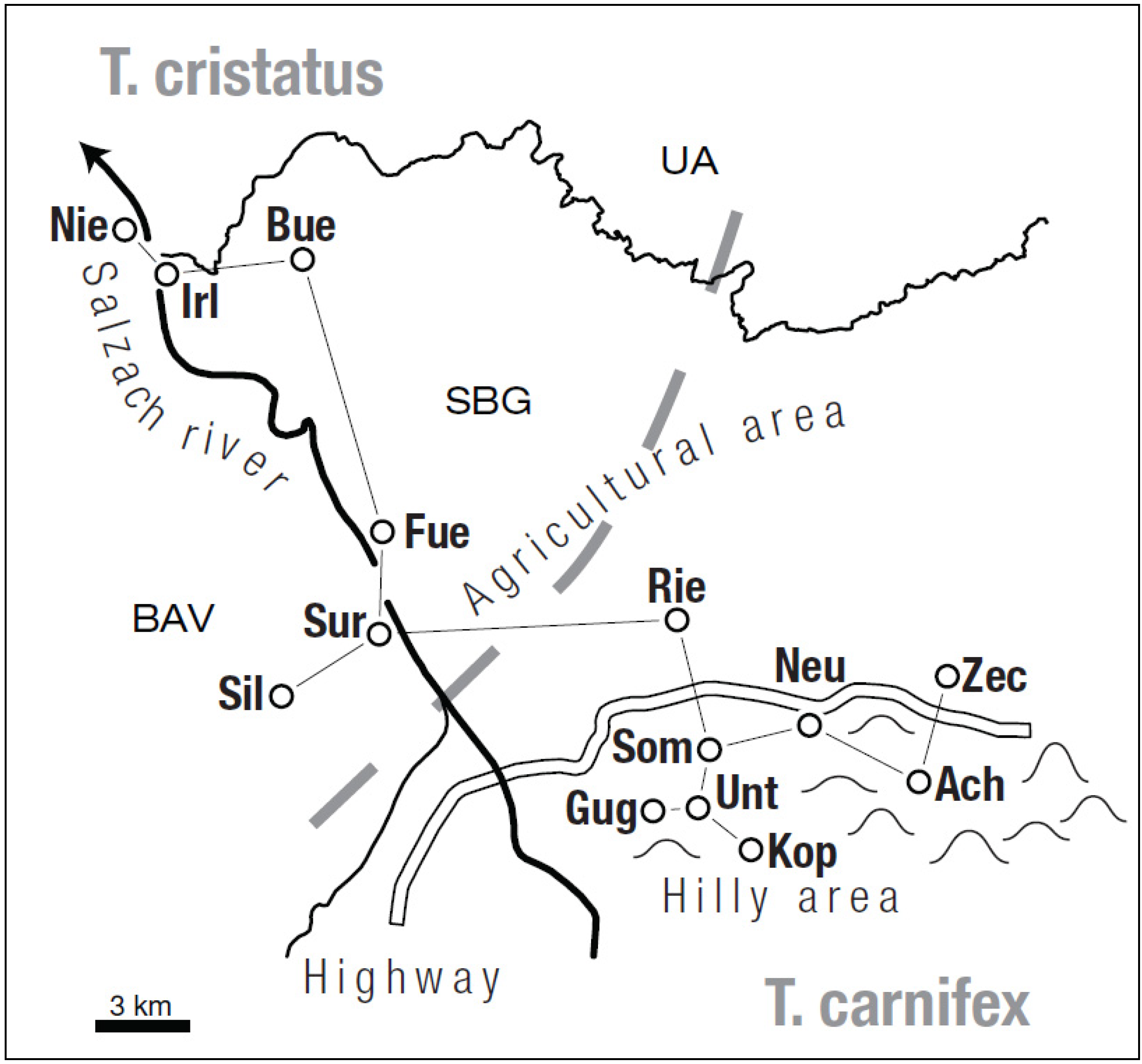
2.1. Study Area and Sample Collection
| Locality | Country | N | Latitude (°N) | Longitude (°E) | |
|---|---|---|---|---|---|
| T. cristatus-like | |||||
| Niederau (Nie) | G | 6 | 48° 00´ | 12° 51´ | |
| Sillersdorf (Sil) | G | 5 | 47° 51´ | 12° 55´ | |
| Surheim (Sur) | G | 7 | 47° 52´ | 12° 58´ | |
| Irlach (Irl) | A | 7 | 47° 59´ | 12° 52´ | |
| Bürmoos (Bue) | A | 16 | 47° 59´ | 12° 56´ | |
| Fürwag (Fue) | A | 17 | 47° 54´ | 12° 58´ | |
| T. carnifex-like | |||||
| Ried (Rie) | A | 20 | 47° 52´ | 13° 06´ | |
| Guggenthal (Gug) | A | 7 | 47° 49´ | 13° 06´ | |
| Unterkoppl (Unt) | A | 7 | 47° 49´ | 13° 07´ | |
| Sommeregg (Som) | A | 18 | 47° 50´ | 13° 07´ | |
| Neuhofen (Neu) | A | 15 | 47° 50´ | 13° 10´ | |
| Koppl (Kop) | A | 2 | 47° 48´ | 13° 08´ | |
| Zecherl (Zec) | A | 11 | 47° 58´ | 13° 14´ | |
| Achleiten (Ach) | A | 7 | 47° 49´ | 13° 13´ |
2.2. DNA-Extraction and Microsatellite Genotyping
2.3. Genetic Diversity
2.4. Population Differentiation and Isolation by Distance Pattern
2.5. Spatial Genetic Structure (Bayesian Approach)
2.6. Spatial Patterns
3. Results and Discussion
3.1. Genetic Diversity within and between Populations
| Locality | He | Ho | Nr. of alleles | Allelic richness | HW (p) | FIS |
|---|---|---|---|---|---|---|
| Nie | 0.48 | 0.48 | 21 | 2.85 | 0.094 | 0.152 |
| Sil | 0.34 | 0.37 | 14 | 2.00 | 0.472 | 0.028 |
| Sur | 0.59 | 0.49 | 29 | 3.62 | 0.004** | 0.228** |
| Irl | 0.58 | 0.49 | 22 | 2.91 | 0.865 | −0.060 |
| Bue | 0.59 | 0.55 | 20 | 3.40 | 0.02* | 0.061* |
| Fue | 0.55 | 0.58 | 26 | 2.95 | 0.254 | −0.019 |
| Rie | 0.57 | 0.5 | 27 | 3.52 | 0.002** | 0.122** |
| Gug | 0.65 | 0.65 | 35 | 2.93 | 0.927 | −0.188 |
| Unt | 0.62 | 0.5 | 34 | 3.13 | 0.503 | −0.006 |
| Som | 0.55 | 0.54 | 25 | 3.33 | 0.044* | 0.106 |
| Neu | 0.63 | 0.51 | 16 | 3.28 | 0.382 | −0.013 |
| Zec | 0.63 | 0.42 | 33 | 3.53 | 0.005* | 0.225** |
| Ach | 0.68 | 0.57 | 21 | 3.75 | 0.001** | 0.255** |
| Nie | Sil | Sur | Irl | Bue | Fue | Rie | Gug | Unt | Som | Neu | Zec | Ach | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nie | 17.6 | 17 | 2 | 6.2 | 14.1 | 24 | 28.1 | 29.2 | 28 | 30.1 | 33.4 | 34.7 | |
| Sil | 0.18 | 4.3 | 15.8 | 15.8 | 6.9 | 14.5 | 14 | 15.6 | 15.5 | 19 | 24 | 23.2 | |
| Sur | 0.01* | 0.09* | 15.1 | 13.8 | 3.6 | 10.6 | 11.6 | 13 | 12.4 | 15.6 | 20.3 | 20 | |
| Irl | 0.33 | 0.52 | 0.16 | 4.9 | 12 | 22.1 | 26.1 | 27.1 | 26 | 26 | 31.3 | 32.7 | |
| Bue | 0.11 | 0.10 | 0.06 | 0.25 | 10.1 | 18.7 | 23.4 | 24.4 | 22.9 | 24.7 | 27.6 | 29 | |
| Fue | 0.12 | 0.21 | 0.04 | 0.23 | 0.04 | 11.1 | 14.1 | 15.1 | 14.2 | 16.8 | 20.9 | 21.3 | |
| Rie | 0.36 | 0.45 | 0.28 | 0.27 | 0.25 | 0.33 | 6.9 | 6.9 | 4.8 | 6 | 9.9 | 10.5 | |
| Gug | 0.30 | 0.64 | 0.33 | 0.26 | 0.41 | 0.49 | 0.13 | 1.7 | 3 | 6.5 | 11.7 | 9.6 | |
| Unt | 0.48 | 0.61 | 0.36 | 0.30 | 0.33 | 0.42 | 0.10 | 0.04 | 2.2 | 4.9 | 10.1 | 7.9 | |
| Som | 0.43 | 0.54 | 0.29 | 0.42 | 0.30 | 0.26 | 0.23 | 0.37 | 0.36 | 3.7 | 9 | 7.6 | |
| Neu | 0.53 | 0.76 | 0.45 | 0.46 | 0.46 | 0.52 | 0.10 | 0.09 | 0.10 | 0.32 | 5.1 | 4.5 | |
| Zec | 0.51 | 0.74 | 0.42 | 0.42 | 0.41 | 0.43 | 0.21 | 0.10 | 0.15 | 0.21 | 0.13 | 3.9 | |
| Ach | 0.35 | 0.63 | 0.24 | 0.34 | 0.36 | 0.37 | 0.04 | 0.06 | 0.06* | 0.20 | 0.03* | 0.06 |
3.2. Spatial Genetic Structure (Bayesian Approach)
| Locality | Structure run 1 Cluster (average q) | Structure run 2 Cluster (average q) | Baps clusters | Geneland clusters | |
|---|---|---|---|---|---|
| T. cristatus- | Nie | 1 (0.992) | 1a (0.966) | 1 | 1 |
| like | Sil | 1 (0.991) | 1a (0.973) | 1 | 1 |
| Sur | 1 (0.971) | 1a (0.901) | 2 | 1 | |
| Irl | 2 (0.857) | 2 (1.000) | 3 | 4 | |
| Bue | 1 (0.975) | 1a (0.890) | 2 | 4 | |
| Fue | 1 (0.988) | 1a (0.916) | 2 | 2 | |
| T. carnifex- | Rie | 2 (0.882) | 2 (1.000) | 4 | 5 |
| like | Gug | 2 (0.985) | 2 (1.000) | 4 | 5 |
| Unt | 2 (0.975) | 2 (1.000) | 4 | 5 | |
| Som | 1 (0.870) | 1b (0.954) | 5 | 3 | |
| Neu | 2 (0.972) | 2 (1.000) | 4 | 3 | |
| Kop | 2 (0.905) | 2 (1.000) | 4 | 3 | |
| Zec | 2 (0.814) | 2 (1.000) | 6 | 6 | |
| Ach | 2 (0.966) | 2 (1.000) | 4 | 6 |


3.3. Spatial Patterns
4. Conclusions
Acknowledgements
References
- Houlahan, J.E.; Findlay, C.S.; Schmidt, B.R.; Meyer, A.H.; Kuzmin, S.L. Quantitative evidence for global amphibian population declines. Nature 2000, 404, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Beebee, T.J.C.; Griffiths, R.A. The amphibian decline crisis: a watershed for conservation biology? Biol. Conserv. 2005, 125, 271–285. [Google Scholar] [CrossRef]
- Beebee, T.J.C.; Wilkinson, J.W.; Buckley, J. Amphibian declines are not uniquely high amongst the vertebrates: trend determination and the British perspective. Diversity 2009, 1, 67–88. [Google Scholar] [CrossRef]
- Diamond, J.M. The present, past and future of human caused extinctions. Philos. T. Roy. Soc. B 1989, 325, 469–477. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evo. S. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Vos, C.C.; Chardon, J.P. Effects of habitat fragmentation and road density on the distribution pattern of the moor frog Rana arvalis. J. Appl. Ecol. 1998, 35, 44–56. [Google Scholar] [CrossRef]
- Andersen, L.W.; Fog, K.; Damgaard, C. Habitat fragmentation causes bottlenecks and inbreeding in the European tree frog (Hyla arborea). P. R. Soc. B 2004, 271, 1293–1302. [Google Scholar] [CrossRef]
- Lesbarrères, D.; Primmer, C.R.; Lodé, T.; Merilä, J. The effects of 20 years of highway presence on the genetic structure of Rana dalmatina populations. Ecoscience 2006, 13, 531–538. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Wake, D.B.; Sousa, W.P. Amphibian declines: judging stability, persistence, and susceptibility of populations to local and global extinctions. Conserv. Biol. 1994, 8, 60–71. [Google Scholar] [CrossRef]
- Marsh, D.M.; Trenham, P.C. Metapopulation dynamics and amphibian conservation. Conserv. Biol. 2001, 15, 40–49. [Google Scholar] [CrossRef]
- Jehle, R.; Sinsch, U. Wanderleistung und Orientierung von Amphibien: eine Übersicht. Zeitschrift für Feldherpetologie 2007, 14, 137–152. [Google Scholar]
- Alford, R.A.; Richards, S.J. Global amphibian declines: a problem in applied ecology. Annu. Rev. Ecol. Syst. 1999, 30, 133–165. [Google Scholar] [CrossRef]
- Smith, M.A.; Green, D.M. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 2005, 28, 110–128. [Google Scholar] [CrossRef]
- Scribner, K.T.; Arntzen, J.W.; Cruddace, N.; Oldham, R.S.; Burke, T. Environmental correlates of toad abundance and population genetic diversity. Biol. Conserv. 2001, 98, 201–210. [Google Scholar] [CrossRef]
- Jehle, R.; Burke, T.; Arntzen, J.W. Delineating fine-scale genetic units in amphibians: probing the primacy of ponds. Conserv. Genet. 2005, 6, 227–234. [Google Scholar] [CrossRef]
- Beebee, T.J.C. Conservation genetics of amphibians. Heredity 2005, 95, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Rowe, G.; Beebee, T.J.C. Defining population boundaries: use of three Bayesian approaches with microsatellite data from British natterjack toads (Bufo calamita). Mol. Ecol. 2007, 16, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. The genetical structure of populations. Annals of Eugenics 1951, 15, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Balloux, F.; Lugon-Moulin, N. The estimation of population differentiation with microsatellite markers. Mol. Ecol. 2002, 11, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Manel, S.; Gaggiotti, O.E.; Waples, R.S. Assignment methods: matching biological questions with appropriate techniques. Trends. Ecol. Evol. 2005, 20, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.B.; Schlötterer, C. Microsatellites: evolution and applications; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Jehle, R.; Arntzen, J.W. Microsatellite markers in amphibian conservation genetics. Herpetol. J. 2002, 12, 1–9. [Google Scholar]
- Arntzen, J.W. Triturus Cristatus Superspezies—Kammmolch-Artenkreis. In Handbuch der Reptilien und Amphibien Europas; Grossenbacher, K., Thiesmeier, B., Eds.; Aula-Verlag: Wiesbaden, Germany, 2003; Volume 4, pp. 422–486. [Google Scholar]
- Jehle, R.; Arntzen, J.W. Post-breeding migrations of newts (Triturus cristatus, T. marmoratus) with contrasting ecological requirements. J. Zool. 2000, 251, 297–306. [Google Scholar]
- Schabetsberger, R.; Jehle, R.; Maletzky, A.; Pesta, J.; Sztatecsny, M. Delineation of terrestrial reserves for amphibians based on post-breeding migration distances: a case study with Italian crested newts (Triturus c. carnifex) at high altitude. Biol. Conserv. 2004, 117, 95–104. [Google Scholar]
- Arntzen, J.W.; Espregueira Themudo, G.; Wielstra, B. The phylogeny of crested newts (Triturus cristatus superspecies): nuclear and mitochondrial genetic characters suggest a hard polytomy, in line with the paleogeography of the centre of origin. Contributions to Zoology 2007, 76, 261–278. [Google Scholar]
- Freytag, G.E. Über Triturus cristatus bei Salzburg (Amphibia: Caudata: Salamandridae). Salamandra 1978, 14, 45–46. [Google Scholar]
- Maletzky, A.; Mikulíček, P.; Franzen, M.; Goldschmid, A.; Gruber, H.-J.; Horak, A.; Kyek, M. Introgression and hybridization and introgression between two species of crested newts (Triturus cristatus and T. carnifex) along contact zones in Germany and Austria: morphological and molecular data. Herpetol. J. 2008, 18, 1–15. [Google Scholar]
- Arntzen, J.W.; Kuzmin, S.; Jehle, R.; Beebee, T.J.C.; Tarkhnishvili, D.; Ishchenko, V.; Ananjeva, N.; Orlov, N.; Tuniyev, B.; Denoël, M.; Nyström, P.; Anthony, B.; Schmidt, B.; Ogrodowczyk, A. Triturus cristatus. IUCN Red List of Threatened Species, IUCN 2009, Version 2009.2; 2008. Available online: www.iucnredlist.org (accessed on 14 December 2009).
- Romano, A.; Arntzen, J.W.; Denoël, M.; Jehle, R.; Andreone, F.; Anthony, B.; Schmidt, B.; Babik, W.; Schabetsberger, R.; Vogrin, M.; Puky, M.; Lymberakis, P.; Crnobrnja-Isailovic, J.; Ajtic, R.; Corti, C. Triturus carnifex. IUCN Red List of Threatened Species, IUCN 2009, Version 2009.2; 2008. Available online: www.iucnredlist.org (accessed on 14 December 2009). [Google Scholar]
- Maletzky, A.; Kyek, M.; Goldschmid, A. Monitoring status, habitat features and amphibian species richness of crested newt (Triturus cristatus superspecies) ponds at the edge of the species range (Salzburg, Austria). Ann. Limnol. – Int. J. Lim. 2007, 43, 107–115. [Google Scholar]
- Kyek, M.; Maletzky, A. Atlas und Rote Liste der Amphibien und Reptilien Salzburgs. Naturschutz-Beiträge 2006, 33, 1–240. [Google Scholar]
- Quantum GIS Development Team. GNU General Public License. 2009. Available online: http://qgis.osgeo.org (accessed on 14 December 2009).
- Pidancier, N.; Miquel, C.; Miaud, C. Buccal swabs as non-destructive tissue sampling method for DNA analysis in amphibians. Herpetol. J. 2003, 13, 175–178. [Google Scholar]
- Broquet, T.; Berset-Braendli, L.; Emaresi, G.; Fumagalli, L. Buccal swabs allow efficient and reliable microsatellite genotyping in amphibians. Conserv. Genet. 2007, 8, 509–511. [Google Scholar] [CrossRef]
- Krupa, A.P.; Jehle, R.; Dawson, D.; Gentle, L.A.; Gibbs, M.; Arntzen, J.W.; Burke, T. Microsatellite loci in the crested newt (Triturus cristatus), and their utility in other taxa. Conserv. Genet. 2001, 3, 87–89. [Google Scholar]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Raymond, M.; Rousset, F. An exact test for population differentiation. Evolution 1995, 49, 1280–1283. [Google Scholar] [CrossRef]
- Hardy, O.J.; Vekemans, X. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (version 1.2): a computer program to calculate F-statistics. J. Hered. 1995, 86, 485–486. [Google Scholar]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Wright, S. Evolution and the genetics of populations. Volume 4—Variability within and among natural populations; University of Chicago Press: Chicago, IL, USA, 1978. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry—The Principles and Practices of Statistics in Biological Research; WH Freeman: New York, NY, USA, 1995. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [PubMed]
- Evanno, G.; Regnault, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: a simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Corander, J.; Waldmann, P.; Sillanpää, M.J. Bayesian analysis of genetic differentiation between populations. Genetics 2003, 163, 367–374. [Google Scholar] [PubMed]
- Corander, J.; Marttinen, P.; Mäntyniemi, S. Bayesian identification of stock mixtures from molecular marker data. Fish. Bull. 2006, 104, 550–558. [Google Scholar]
- Guillot, G.; Estoup, A.; Mortier, F.; Cosson, J.F. A spatial statistical model for landscape genetics. Genetics 2005, 170, 1261–1280. [Google Scholar] [CrossRef] [PubMed]
- Guillot, G.; Mortimer, F.; Estoup, A. Geneland: a computer program for landscape genetics. Mol. Ecol. Notes 2005, 5, 712–715. [Google Scholar] [CrossRef]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org (accessed on 14 December 2009).
- Coulon, A.; Guillot, G.; Cosson, J.-F.; Angibault, M.A.; Aulagnier, S.; Cargnelutti, B.; Galan, M.; Hewison, A.J.M. Genetic structure is influenced by landscape features: empirical evidence from a roe deer population. Mol. Ecol. 2006, 15, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, G.T. The relative neighborhood graph of a finite planar set. Pattern Recogn. 1980, 12, 261–268. [Google Scholar] [CrossRef]
- Kupfer, A. Wanderstrecken einzelner Kammmolche (Triturus cristatus) in einem Agrarlebensraum. Zeitschrift für Feldherpetologie 1998, 5, 238–242. [Google Scholar]
- Miaud, C.; Joly, P.; Castanet, J. Variation in age structures in a subdivided population of Triturus cristatus. Can. J. Zoolog. 1993, 71, 1874–1879. [Google Scholar] [CrossRef]
- Mikulíček, P. Hybridization and genetic differentiation of the crested newts (triturus cristatus superspecies) in central europe—analysis of nuclear markers. PhD thesis, University of Prague, Czech Republic, 2005. [Google Scholar]
- Jehle, R.; Arntzen, J.W.; Burke, T.; Krupa, A.P.; Hödl, W. The annual number of breeding adults and the effective population size of syntopic newts (Triturus cristatus, T. marmoratus). Mol. Ecol. 2001, 10, 839–850. [Google Scholar]
- Meyer, S.; Grosse, W.-R. Populationsgröße, Altersstruktur und genetische Diversität einer Metapopulation des Kammmolches (Triturus cristatus) in der Kulturlandschaft Sachsen-Anhalts. Zeitschrift für Feldherpetologie 2007, 14, 9–24. [Google Scholar]
- Arens, P.; Bugter, R.; van´t Westende, W.; Zolliner, R.; Stronks, J.; Vos, C.C.; Smulders, M.J.M. Microsatellite variation and population structure of a recovering Tree frog (Hyla arborea L.) metapopulation. Conserv. Genet. 2006, 7, 825–834. [Google Scholar]
- Garner, T.W.J.; Angelone, S.; Pearman, P.B. Genetic depletion in Swiss populations of Rana latastei: conservation implications. Biol. Conserv. 2003, 114, 371–376. [Google Scholar] [CrossRef]
- Kraaijeveld-Smit, F.J.L.; Beebee, T.J.C.; Griffiths, R.A.; Moore, R.D.; Schley, L. Low gene flow but high genetic diversity in the threatened Mallorcan midwife toad Alytes muletensis. Mol. Ecol. 2005, 14, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Kalezić, M.L.; Hedgecock, D. Genetic variation and differentiation of three common European newts (Triturus) in Yugoslavia. Brit. J. Herpetol. 1980, 6, 49–57. [Google Scholar]
- Castric, V.; Bernatchez, L. Individual assignment test reveals differential restriction to dispersal between two salmonids despite no increase of genetic differences with distance. Mol. Ecol. 2004, 13, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Waples, R.S.; Gaggiotti, O. What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol. Ecol. 2006, 15, 1419–1439. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.M.; Verkenne, C.; Vandewoestijne, S.; Wesselingh, R.A.; Baguette, M. Gene flow and functional connectivity in the natterjack toad. Mol. Ecol. 2006, 15, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Manel, S.; Segelbacher, G. Perspectives and challenges in landscape genetics. Mol. Ecol. 2009, 18, 1821–1822. [Google Scholar] [CrossRef] [PubMed]
- Halley, J.M.; Oldham, R.S.; Arntzen, J.W. Predicting the persistence of amphibian populations with the help of a spatial model. J. Appl. Ecol. 1996, 33, 455–470. [Google Scholar] [CrossRef]
- Maletzky, A.; Goldschmid, A.; Kyek, M. Crested newt (Triturus cristatus superspecies) populations in Salzburg, Austria, their distribution, size and conservation status. Herpetozoa 2008, 20, 145–163. [Google Scholar]
- Hels, T.; Buchwald, E. The effect of road kills on amphibian populations. Biol. Conserv. 2001, 99, 331–340. [Google Scholar] [CrossRef]
- Hitchings, S.P.; Beebee, T.J.C. Genetic substructuring as a result of barriers to gene flow in urban Rana temporaria (common frog) populations: implications for biodiversity conservation. Heredity 1997, 79, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Pellet, J.; Guisan, A.; Perrin, N. A concentric analysis of the impact of urbanization on the threatened European tree frog in an agricultural landscape. Conserv. Biol. 2004, 18, 1599–1606. [Google Scholar] [CrossRef]
- Williams, P.; Biggs, J.; Whitfield, M.; Thorne, A.; Bryant, S.; Fox, G.; Nicolet, P. The Pond Book: a Guide to the Management and Creation of Ponds; Ponds Conservation Trust: Oxford, England, 1999. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maletzky, A.; Kaiser, R.; Mikulíček, P. Conservation Genetics of Crested Newt Species Triturus cristatus and T. carnifex within a Contact Zone in Central Europe: Impact of Interspecific Introgression and Gene Flow. Diversity 2010, 2, 28-46. https://doi.org/10.3390/d2010028
Maletzky A, Kaiser R, Mikulíček P. Conservation Genetics of Crested Newt Species Triturus cristatus and T. carnifex within a Contact Zone in Central Europe: Impact of Interspecific Introgression and Gene Flow. Diversity. 2010; 2(1):28-46. https://doi.org/10.3390/d2010028
Chicago/Turabian StyleMaletzky, Andreas, Roland Kaiser, and Peter Mikulíček. 2010. "Conservation Genetics of Crested Newt Species Triturus cristatus and T. carnifex within a Contact Zone in Central Europe: Impact of Interspecific Introgression and Gene Flow" Diversity 2, no. 1: 28-46. https://doi.org/10.3390/d2010028




