Review of the Hygrophilous Weevils in Israel (Coleoptera: Curculionoidea)
Abstract
:1. Introduction
2. Materials and Methods
- BMNH—Natural History Museum, London, UK (M. Barclay).
- HNHM—Hungarian Natural History Museum, Budapest, Hungary.
- USNM—National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (L. Chamorro).
- ZSMU—Zoologische Staatssamlung München, Munich, Germany (M. Balke).
3. Taxonomy
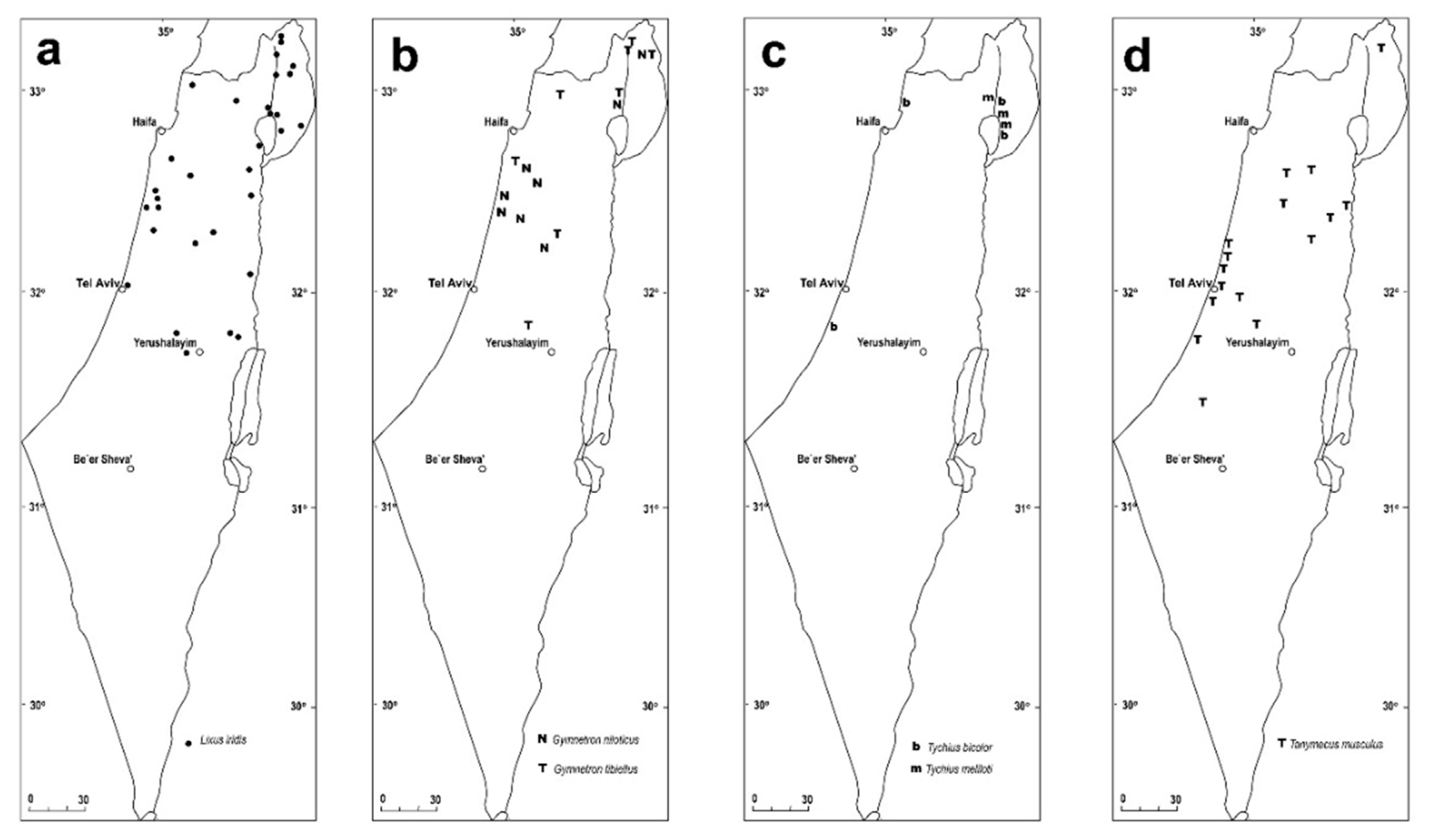
3.1. List of the Hygrophilous Weevils of Israel
3.2. Identification Keys to the Hygrophilous Weevils in Israel
- 1.
- Trochanter oblong, at least twice as long as wide, base of femur not touching coxa ........... Brentidae 2
- -
- Trochanter short, at most as long as wide, base of femur touching coxa ............ Curculionidae 8
- 2.
- -
- Antenna geniculate, scapus longer than or as long as funicle, funicle 4- or 5-segmented; body globular, yellow to light brown; bases of pronotum and elytra crenulate; pronotum at base as wide as elytra (5b-h, 6c-i); on Lythrum salicaria or Tamarix ......... Nanophyinae 3
- 3.
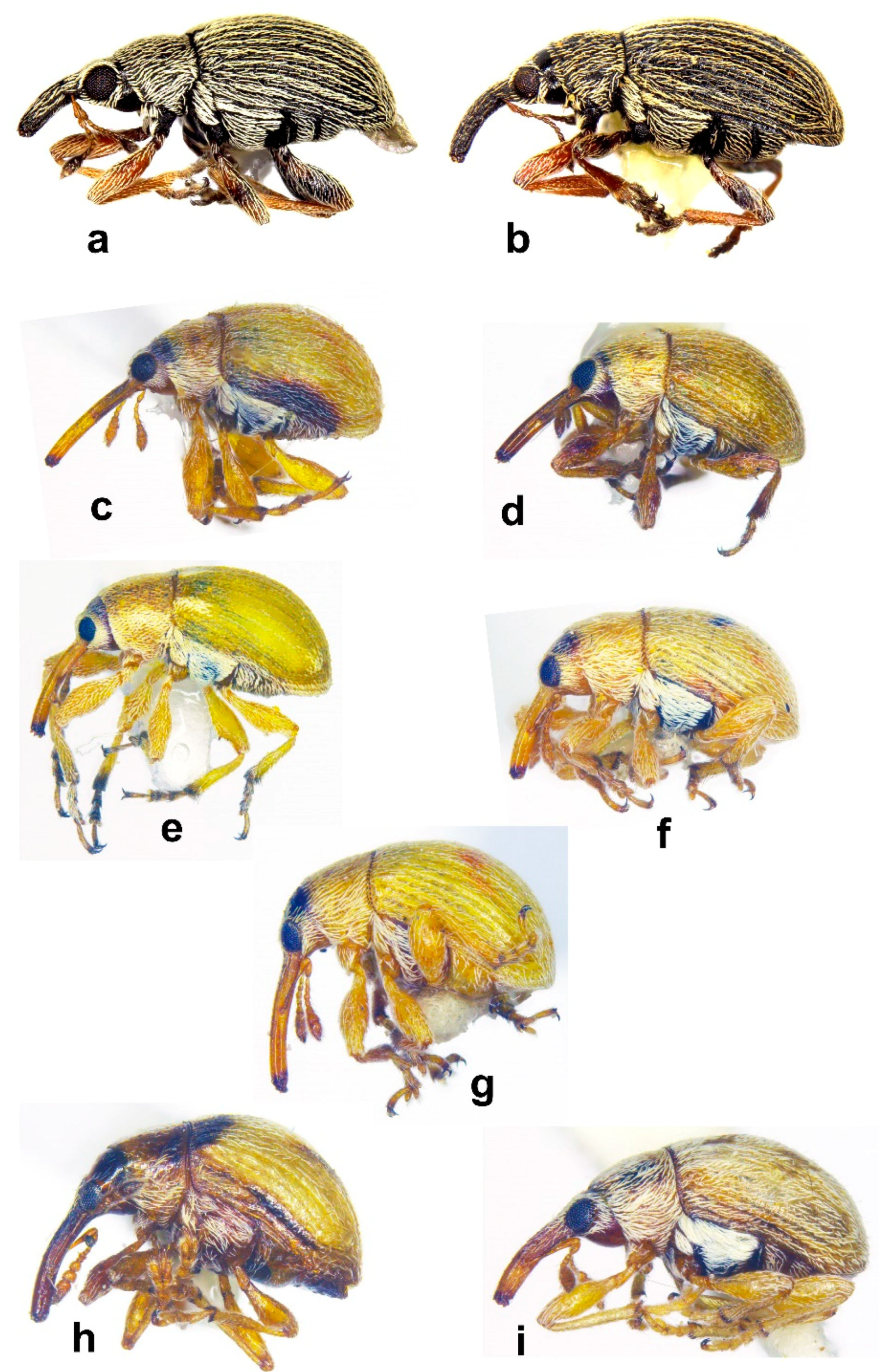
- Antennal club compact, comprised of fused segments with sutures distinctly visible, obsolete or invisible (5b–f, 6c–g); male tibia not mucronate; claws free; on Tamarix (Figure 1a–d) .......... Corimaliini 4
- -
- Antennal club loose, comprised of distinctly separated segments; male tibia mucronate; claws fused at least at base; on Lythrum (Figures 1e, 2c and 3d) ......... Nanophyini 6
- 4.
- Antennal funicle 5-segmented, antennal club with distinct sutures ......... Corimalia
- -
- Antennal funicle 4-segmented, antennal club with distinct or indistinct sutures .......... 5
- 5.
- Antennal club with distinct sutures; elytra distinctly crenulate at base; femora with two denticles; if with one denticle only, then denticle distinctly longer than wide; body length 1.5–2.0 mm .......... Allomalia
- -
- Antennal club without distinct sutures; elytra not crenulate at base; femora with one denticle or without denticles; body length 0.8–1.5 mm ........ Hypophyes
- 6.
- 8th elytral interstria completely not crenulate; male pygidium apically with round fovea; femora without denticles or (rarely) with minute denticle ........ Nanophyes
- -
- 8th elytral interstria crenulate at least close to base; male pygidium without fovea; femora dentate ........ 7
- 7.
- 8th elytral interstria crenulate at basal quarter; femora strongly incrassate, with large proximal and small distal denticles ........ Dieckmanniellus
- -
- 8th elytral interstria crenulate between base and humeral callus; femora slightly incrassate, with one minute denticle or (rare) without denticle ...... Nanomimus
- 8.
- Claws dentate; mesothoracic epimeron visible in dorsal view; rostrum as long as pronotum, thick, hidden in rostral channel; body stout, 1.5–4.0 mm (Figure 12a–d) ...... Rhinoncus 9
- -
- Claws not dentate; mesothoracic epimeron not visible in dorsal view; rostrum and body of various shapes and size, rostral channel present or absent ........ 10
- 9.
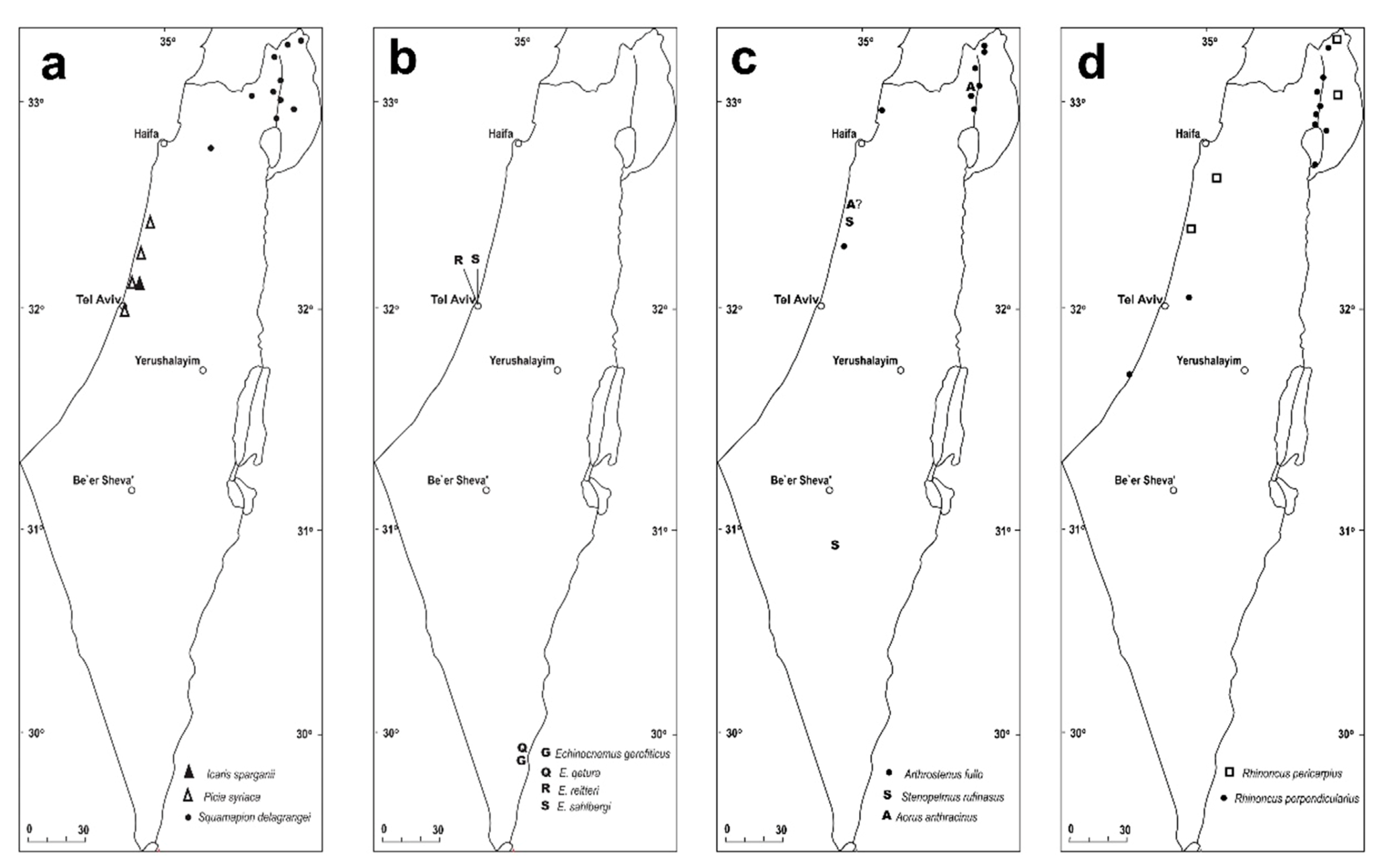
- Claw denticle short, 0.3X as long as claw, straight, not contiguous to opposite claw denticle at apex; body laterally rounded, black, with slight bluish tinge, body length 2.5–4.0 mm (Figure 12a,c) ......... Rhinoncus pericarpius
- -
- Claw denticle long, 0.5X as long as claw, curved, nearly contigous to oposit claw denticle at apex; body laterally subparallel, testaceous to dark brown, body length 1.5–2.0 mm (Figure 12b,d) ........ Rhinoncus perpendicularis
- 10.
- Antennal funicle 5-segmented; on Veronica anagallis-aquatica (Figure 2d,e) ........ Gymnetron 11
- -
- Antennal funicle 7-segmented ........ 12
- 11.
- Scales on elytral interstriae denser, arranged in three irregular rows (Figure 14e); male rostrum strongly tapering at apex, slightly turned up at apex in lateral view (Figure 14a), female rostrum slightly evenly curved (Figure 14b); body length 1.7–2.0 mm ........ Gymnetron niloticum
- -
- Scales on elytral interstriae sparser, arranged in one regular row (Figure 14f); male rostrum slightly tapering at apex, slightly evenly bent, not turned up at apex in lateral view (Figure 14c), female rostrum nearly straight (Figure 14d); body length 1.5–1.8 mm ........ Gymnetron tibiellum
- 12.
- Rostrum as long as wide or shorter, shorter than pronotum ........ 13
- -
- Rostrum at least 1.5X as long as wide, as long as or longer than pronotum ........ 15
- 13.
- Rostrum as long as wide; 3rd tarsomere narrow, as wide as 2nd, with lobes separated in distal half only; abdomen segments covered with round scales; body stout, 1.6–2.0 mm long (Figure 10d,e) ........ Stenopelmus rufinasus
- -
- Rostrum shorter than wide; 3rd tarsomere distinctly wider than 2nd, entirely divided into two rounded lobes; abdomen covered with either piliform scales or combination of round and piliform scales; body oblong, 3.0–7.0 mm long ........ 14
- 14.
- Fore margin of pronotum laterally without postocular tuft of setae; mandibles without mandibular cusp or round scar; body covered by appressed scales only, laterally with wide, pale lateral stripe of white scales (Figure 12g,i) ........ Sitona lividipes
- -
- Fore margin of pronotum laterally with postocular tuft of setae; mandibles without mandibular cusp or round scar; body covered by appressed, erect and semi-erect scales, unicolorous, laterally without pale lateral stripe of scales (Figure 12h,j) ........ Tanymecus musculus
- 15.
- Rostrum anteriorly tapering at least from antennal insertion, apically pointed; on Melilotus albus ........ Tychius 16
- -
- Rostrum cylindrical, at most slightly widened apically ........ 17
- 16.
- Metafemur with anteromedian denticle, male protibia without denticle medially; rostrum in lateral view less curved at base and gradually tapering towards apex; upper part of body covered with yellowish scales, scales truncated apically (Figure 14g,i–k) ........ Tychius bicolor
- -
- All femora without denticles, male protibia with strong denticle medially; rostrum in lateral view strongly curved at base and strongly tapering from antennal insertion place toward apex; upper part covered with whitish scales, scales pointed apically (Figure 14h,l–n) ........ Tychius meliloti
- 17.
- Elytra apically produced into divergent pointed processes; body oblong, laterally subparallel, 3.8–4.0X as long as wide (Figure 12f,k) ........ Lixus iridis
- -
- Elytra apically not produced; body of various shapes, at most 3.3X as long as wide ........ 18
- 18.
- Rostral channel absent; postocular lobes of pronotum absent, at most fore margin of pronotum laterally slightly rounded; antennal sulci covered by scales or bare ........ 19
- -
- Rostral channel present; postocular lobes distinct; antennal sulci bare ........ 25
- 19.
- Body black, slender, oblong, smooth and shiny, completely bare (Figure 10g and Figure 11h,i) ........ Aorus anthracinus
- -
- Body of various color, shape and texture, covered densely by scales ........ 20
- 20.
- 3rd tarsomere cordate; rostrum slender, 4X as long as wide (Figures 10b and 11a) ........ Icaris sparagnii
- -
- 3rd tarsomere narrow, sub-cylindrical, as wide as 2nd at apex; rostrum stout, 2X as long as wide ........ 21
- 21.
- Tarsomeres 1st–3rd slender, oblong, above 2X as long as wide, 3rd not bilobed distally; elytra laterally parallel at basal two thirds, strongly acuminate in apical third (Figures 10c and 11b) ........ Picia syriaca
- -
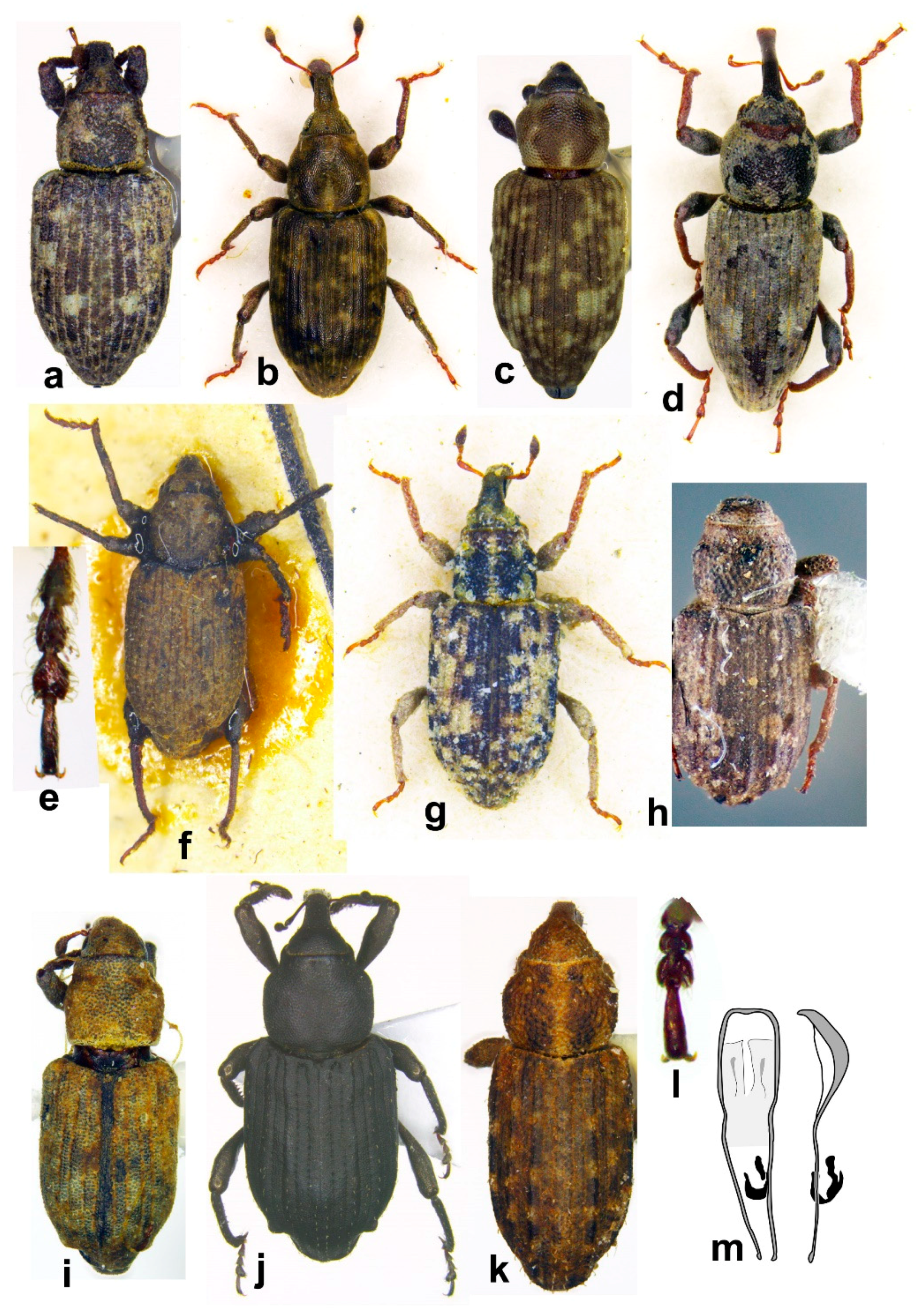
- Tarsomeres 1st–3rd robust, short, at most 1.3X as long as wide, 3rd scarcely to distinctly bilobed distally; elytra parallel at basal 4/5, at apical 1/5 weakly acuminate (Figures 10a and 11c) ........ Echinocnemus
- 22.
- Extant ........ 23
- -
- Fossil ........ 24
- 23.
- Rostrum moderately and evenly curved; tarsi short, 1st tarsomere as long as wide, 2nd–3rd tarsomeres wider than long, 3rd tarsomere distinctly wider than 2nd, bilobed at apex; body length 3.2 mm (Figures 10a and 11c) ........ Echinocnemus reitteri
- -
- Rostrum straight; tarsi oblong, 1st–3rd tarsomeres longer than wide, equal in length and width; body length 4.5–6.5 mm ........ Echinocnemus sahlbergi
- 24.
- Elytron narrower, 5.1 mm long, uniformly colored ........ Echinocnemus qetura
- -
- Elytron wider, 4.5 mm long, with wide transverse bands ........ Echinocnemus gerofiticus
- 25.
- Head constricted posterior to eyes, eyes prominent, antennal sulci ventral; body covered by oblong apically truncated attached scales (Figures 10f and 11d–g) ........ Arthrostenus fullo
- -
- 1.
- Tarsal segments 1–3 transverse or at most as long as wide; 7th segment of flagellum as as wide as 6th, or slightly wider, pubescent in same extent; geophile (collected by sifting or in pitfalls) (Figures 7k–m and 8j,k) ........ libanicus
- -
- Tarsal segments 1–3 longer than wide; 7th segment of flagellum distinctly wider than 6th, more densely pubescent; either aquatic or geophiles ........ 2
- 2.
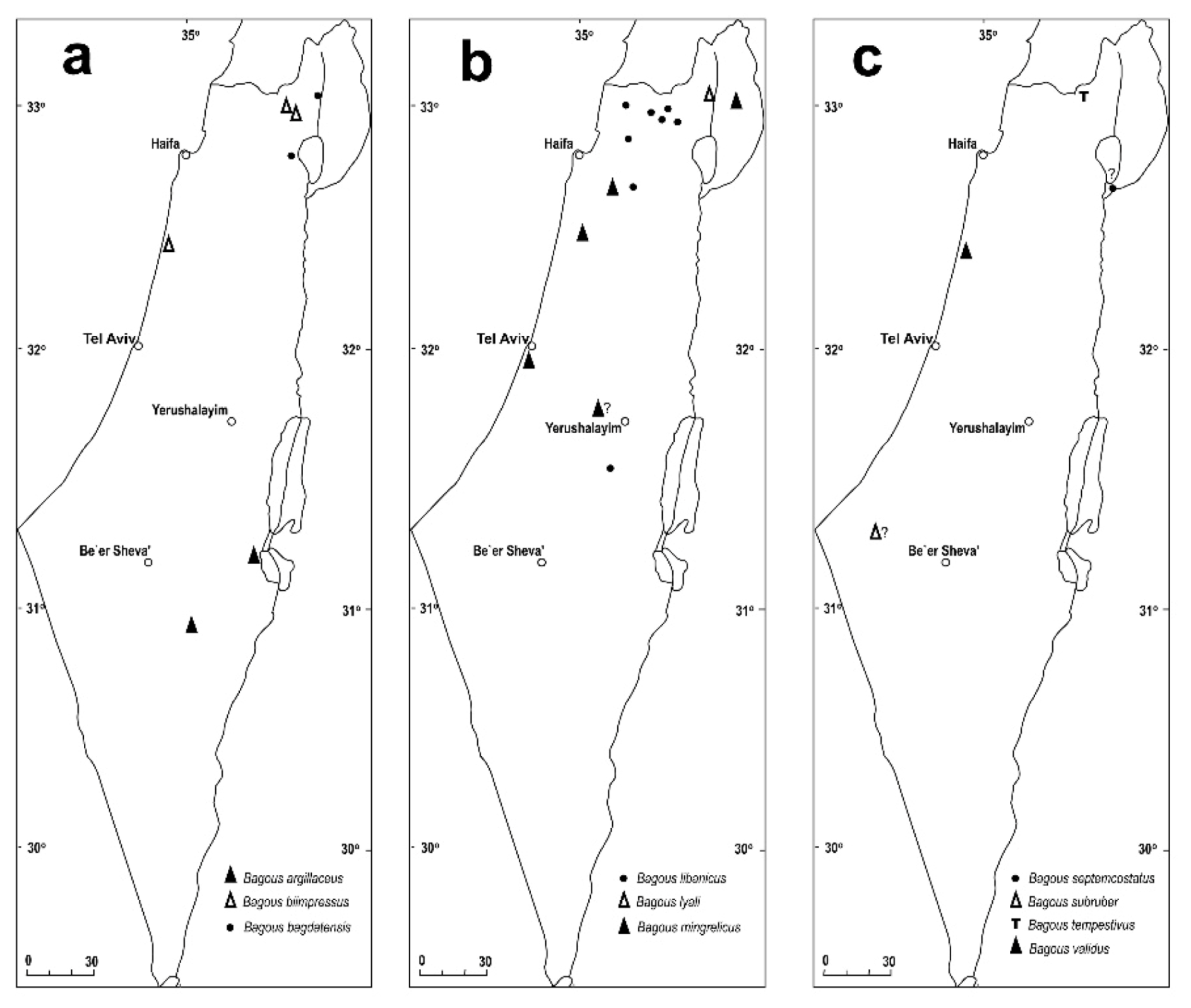
- 1st segment of antennal club bare, smooth, shiny (Figure 8c) ........ biimpressus
- -
- 1st segment of antennal club pubescent, not shiny ........ 3
- 3.
- Tarsal segments cylindrical or nearly so, as long as wide or slightly longer; 3rd tarsal segment not or slightly wider than 2nd tarsal segment at apex ........ 4
- -
- Tarsal segments trapezoidal, distinctly longer than wide; 3rd tarsal segment cordate or at least distinctly wider than 2nd tarsal segment at apex ........ 6
- 4.
- Tarsal segments narrowly trapezoidal; body covered with round smooth shiny scales (Figures 7b and 8b) ........ argillaceus
- -
- Tarsal segments cylindrical; scales not smooth and shiny ........ 5
- 5.
- Pronotum granulate; elytral disc flat, no elytral intervals convex; body elongate, slender, legs long, slender (Figures 7i and 8h); aquatic ........ tempestivus
- -
- Pronotum punctate; elytral disc moderately convex, odd-numbered elytral intervals convex; body rounded, stout, legs short, stout; geophilous ........ 6
- 6.
- Pronotum with small punctures, intervals between punctures distinctly convex, granulose, larger than punctures; elytra subquadrate, 1.30X as long as wide; tarsomere 2 and 3 subglobose, nearly as wide as long; antennae dark brown (Figures 7g and 8f) ........ subruber
- -
- Pronotum with large punctures, intervals between punctures more or less convex, narrower than punctures: elytra more rectangular, 1.40–1.55X as long as wide; tarsomere 2 and 3 slightly but distinctly longer than wide; antennae dark reddish (Figures 7h and 8g) ........ septemcostatus
- 7.
- Pronotum rounded laterally, nearly as wide as elytra; body narrow, 3X as long as wide; body length 3 mm (Figures 7d and 8d) ........ mingrelicus
- -
- Pronotum subparallel laterally, distinctly narrower than elytra; body oblong, 2.25–2.50X as long as wide ........ 8
- 8.
- 3rd tarsomere narrow, as long and as wide as 1st and 2nd, or only slightly larger, more or less cordate; body length 2.2–4.5 mm ........ 9
- -
- 3rd tarsomere wide, at least 1.5 as long and as wide as 1st and 2nd, distinctly cordate, lobes separated at one third of its length, 5.5–7.0 mm (Figures 7j and 8j) ........ validus
- 9.
- 3rd tarsomere cordate, lobes separated at least at one third of its length, tarsomeres bare or covered at ventral part by short scales; body length 2.2–2.7 mm (Figures 7a and 8a) ........ bagdatensis
- -
- 3rd tarsomere subcordate, lobes separated at most at one fifth of its length, tarsomeres covered densely by long thin erect scales, partly piliform and partly wider; body length 3.7–4.5 mm (Figures 7e,f and 8e) ........ lyali
3.3. Treatment of Genera and Species
4. Discussion
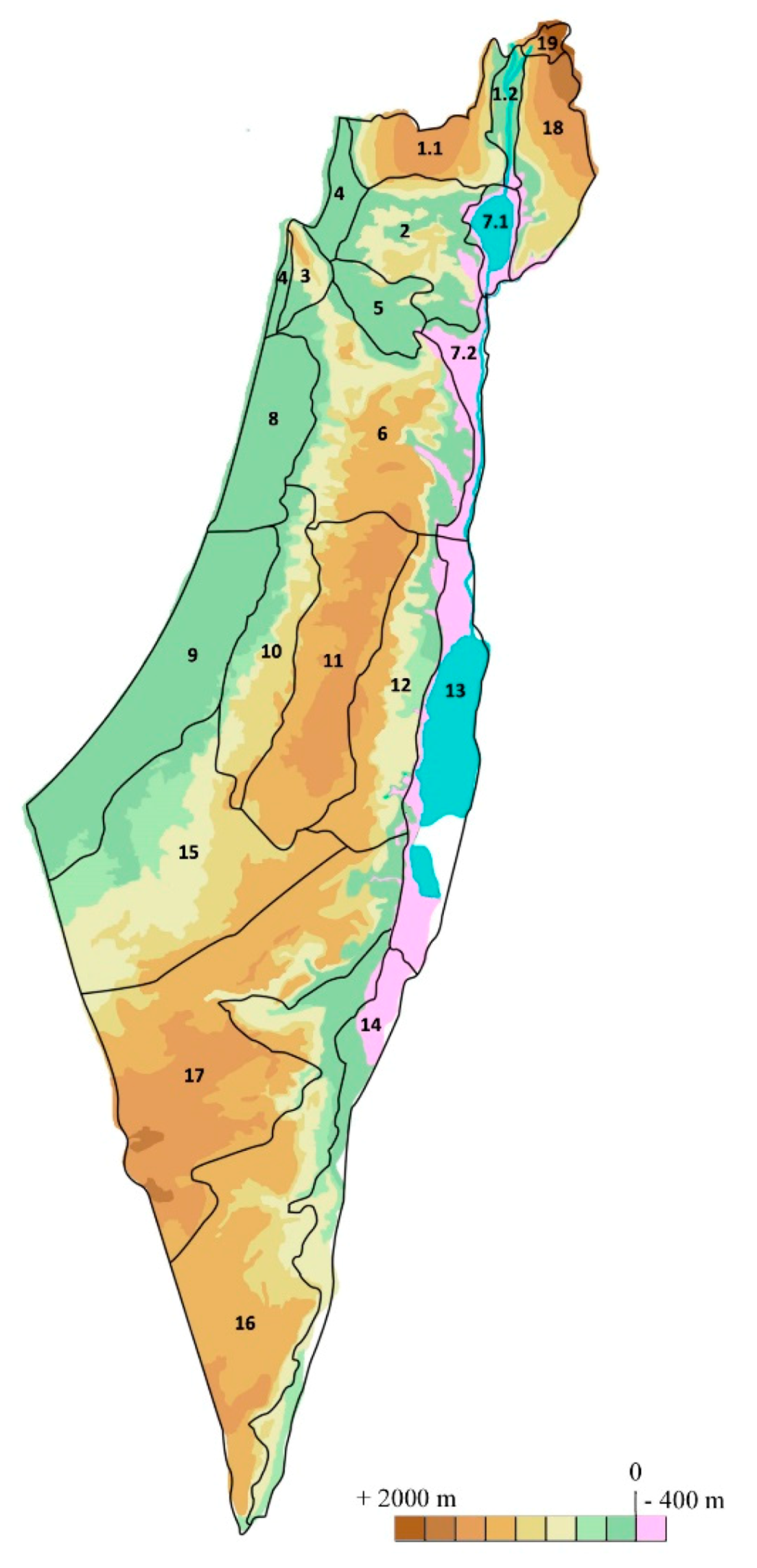
| 1. Upper Galilee |
| 1.1. Upper Galilee Hills |
| 1.2. Hula Valley |
| 2. Lower Galilee |
| 3. Karmel (Carmel) Ridge |
| 4. Northern Coastal Plain |
| 5. Yizre’el (Jezreel) Valley |
| 6. Shomeron (Samaria) |
| 7. Jordan Valley & Southern Golan |
| 7.1. Sea of Galilee Area |
| 7.2. Jordan Valley |
| 8. Central Coastal Plain |
| 9. Southern Coastal Plain |
| 10. Judean Foothills |
| 11. Judean Hills |
| 12. Judean Desert |
| 13. Dead Sea area |
| 14. ‘Arava Valley |
| 15. Northern Negev |
| 16. Southern Negev |
| 17. Central Negev |
| 18. Golan Heights |
| 19. Mount Hermon |
Funding
Acknowledgments
Conflicts of Interest
References
- Oberprieler, R.G.; Marvaldi, A.E.; Anderson, R.S. Weevils, weevils, weevils everywhere. Zootaxa 2007, 1668, 491–520. [Google Scholar]
- Caldara, R.; O’Brien, C.W.; Meregalli, M. A phylogenetic analysis of the aquatic weevil tribe Bagoini (Coleoptera: Curculionidae) based on morphological characters of adults. Zootaxa 2017, 4287, 1–63. [Google Scholar] [CrossRef]
- Egorov, A.B.; Korotyaev, B.A. Review of the weevil tribe Emphyastini (Coleoptera, Curculionidae), habitants on supralittoral of the Sea of Japan, Ochotian and Behring seas. Proc. Zool. Inst. Acad. Sci. USSR 1976, 77, 43–55. (In Russian) [Google Scholar]
- Friedman, A.L.L. Review of the biodiversity and zoogeographical patterns of the weevils (Coleoptera, Curculionoidea) in Israel. ZooKeys 2009, 31, 133–148. [Google Scholar] [CrossRef]
- Kuschel, G. The foreign Curculionoidea established in New Zealand (Insecta: Coleoptera). N. Z. J. Sci. 1972, 15, 273–289. [Google Scholar]
- Oevering, P.; Pitman, A.J. Characteristics of attack of coastal timbers by Pselactus spadix (Herbst) (Col.: Curc.: Cossoninae) and an investigation of its life history. Holzforschung 2002, 56, 335–359. [Google Scholar] [CrossRef]
- Caldara, R.; O’Brien, C.W. Curculionidae: Aquatic weevils of China (Coleoptera). In Water Beetles of China; Jäch, M.A., Ji, L., Eds.; Zoologisch-Botanische Gesellschaft in Österreich and Wiener Coleopterologenverein: Wien, Austria, 1995; pp. 389–408. [Google Scholar]
- Survey of Israel. Israel Touring Map (1:250,000) and List of Settlements (English Version); State of Israel, Ministry of Labor, 2 Sheets; Survey of Israel: Tel Aviv, Israel, 2009. [Google Scholar]
- Israel Meterological Services, Ministry of Transport (IMS). 2018. Available online: http://www.ims.gov.il/IMS/CLIMATE/LongTermRain/ (accessed on 16 May 2018).
- Goren, M.; Ortal, R. Biogeography, diversity and conservation of the inland water fish communities in Israel. Biol. Conserv. 1999, 89, 1–9. [Google Scholar] [CrossRef]
- Por, D. An outline of the Zoogeography of the Levant. Zool. Scr. 1975, 4, 5–11. [Google Scholar] [CrossRef]
- Shmida, A.; Aronson, J.A. Sudanian elements in the flora of Israel. Ann. Mo. Bot. Gard. 1986, 73, 1–28. [Google Scholar] [CrossRef]
- Waisel, Y.; Liphschitz, N. Water Plants in Israel; Nature Conservation Authority: Tel Aviv, Israel, 1973; p. 82. (In Hebrew) [Google Scholar]
- Waisel, Y.; Cohen, Y.; Pollak, G. Ecology of the Vegetation of Israel; Tel Aviv University. Sifriat Poalim: Tel Aviv, Israel, 1982; p. 462. (In Hebrew) [Google Scholar]
- Biton, R.; Geffen, E.; Vences, M.; Cohen, O.; Bailon, S.; Rabinovich, R.; Malka, Y.; Oron, T.; Boistel, R.; Brumfeld, V.; et al. The rediscovered Hula painted frog is a living fossil. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Dumont, H.J. Odonata of the Levant. Fauna Palaestina: Insecta V; The Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1991; p. 300. [Google Scholar]
- Furth, D.G. The Huleh and its lost aquatic leaf beetle. Atala 1976, 4, 4–9. [Google Scholar]
- Dimentman, C.; Bromley, H.J.; Por, F.D. Lake Hula. Reconstruction of the Fauna and Hydrobiology of a Lost Lake; The Israel Academy of Sciences and Humanities: Jerusalem, Israel; Keter Press Enterprises: Jerusalem, Israel, 1992; p. 196. [Google Scholar]
- Hambright, K.D.; Zohary, T. Lakes Hula and Agmon: Destruction and creation of wetland ecosystems in northern Israel. Wetl. Ecol. Manag. 1998, 6, 83–89. [Google Scholar] [CrossRef]
- Karmon, Y.; Livne, M. The Hula Valley and its vicinity. Ariel 1990, 75–76, 1–192. [Google Scholar]
- Freidberg, A. Life in the Dead Sea. Isr. J. Ent. 1981, 15, 101–102. [Google Scholar]
- Ortal, R.; Gabai, S.; Frankenberg, E. National Report on the Implementation of the RAMSAR Convention on Wetlands. Israel. National Reports to Be Submitted to the 10th Meeting of the Conference of the Contracting Parties, Republic of Korea, 28 October–4 November 2008; p. 37. Available online: http://www.sviva.gov.il/InfoServices/ReservoirInfo/DocLib2/Publications/P0401-P0500/P0473.pdf (accessed on 20 May 2018).
- Gasith, A. Conservation and management of the coastal streams of Israel: An assessment of stream status and prospect for rehabilitation. In River Conservation and Management; Boon, P.J., Calow, P., Petts, G.E., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 1992; pp. 51–63. [Google Scholar]
- Levin, N.; Lahav, H.; Ramon, U.; Heller, A.; Nizry, G.; Tsoar, A. Landscape continuity analysis: A new approach to conservation planning in Israel. Landsc. Urban Plan. 2007, 79, 53–64. [Google Scholar] [CrossRef]
- Levin, N.; Elron, E.; Gasith, A. Decline of wetland ecosystems in the coastal plain of Israel during the 20th century: Implications for wetland conservation and management. Landsc. Urban Plan. 2009, 92, 220–232. [Google Scholar] [CrossRef]
- Furth, D.G. Aquatic entomofauna of a Dead Sea oasis. Hydrobiologia 1983, 102, 3–25. [Google Scholar] [CrossRef]
- Botosaneanu, L.; Gasith, A. Contributions taxonomiques et ecologiques a la connaissance des trichopteres (Insecta) d’Israel. Isr. J. Zool. 1971, 20, 89–129. [Google Scholar]
- Bromley, H.J. A note on the Plecoptera of Israel. Isr. J. Ent. 1988, 22, 1–12. [Google Scholar]
- Demoulin, G. Contribution à l’étude des Ephéméroptères d’Israel. Introduction et I. Heptageniidae. Bull. Inst. R. Sci. Nat. Belg. 1973, 49, 1–19. [Google Scholar]
- Lyal, C.H.C. 3.7.7. Molytinae Schoenherr, 1823. pp. 529–570. In Handbook of Zoology. Arthropoda: Insecta; Coleoptera, Beetles. Vol. 3. Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2014; Volume 3, p. 675. [Google Scholar]
- Sartori, M. Mayflies from Israel (Insecta; Ephemeroptera) I. Heptageniidae, Ephemerellidae, Leptophlebiidae & Palingeniidae. Rev. Suisse Zool. 1992, 99, 835–858. [Google Scholar]
- Hebauer, F. The Hydrophiloidea of Israel and the Sinai (Coleoptera, Hydrophiloidea). Zool. Middle East 1994, 10, 73–137. [Google Scholar] [CrossRef]
- Jäch, M.A.; Ortal, R. The large water beetles of Israel—On the verge of extinction. Soc. Prot. Nat. (Israel) 1988, 6, 37–49. [Google Scholar]
- Wewalka, G. Die Arten der Gattung Scarodytes aus Griechenland und eine neue Art dieser Gattung aus Israel (Dytiscidae, Col.). Kol. Rundsch. 1977, 53, 137–144. [Google Scholar]
- Wewalka, G. Systematic and faunistic notes on Noteridae and Dytiscidae of the Near East (Coleoptera). Kol. Rundsch. 1989, 59, 143–152. [Google Scholar]
- Caldara, R.; O’Brien, C.W. Systematics and evolution of Weevils of the genus Bagous. VI. Taxonomic treatment of the species of the Western Palearctic Region (Col. Curculionidae). Mem. Soc. Ent. It. 1998, 76, 131–347. [Google Scholar]
- Alonso-Zarazaga, M.A.; Lyal, C.H.C. A World Catalogue of Families and Genera of Curculionoidea (Insecta: Coleoptera) (Excepting Scolytidae and Platypodidae); Entomopraxis, S.C.P.: Barcelona, Spain, 1999; p. 315. [Google Scholar]
- Danin, A.; Fragman-Sapir, O. Flora of Israel Online. 2017. Available online: http://flora.org.il/en/plants (accessed on 12 June 2018).
- Theodor, O. Diptera Pupipara. Fauna Palaestina. Insecta I; The Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1975; p. 170. [Google Scholar]
- Ionescu, A.; Eyer, P.-A. Notes on Cataglyphis Foerster, 1850 of the bicolor species-group in Israel, with description of a new species (Hymenoptera: Formicidae). Isr. J. Ent. 2016, 46, 109–131. [Google Scholar]
- Caldara, R. Curculionidae: Bagoinae, pp. 172–176. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Brill: Leiden, The Netherlands, 2013; Volume 8, p. 700. [Google Scholar]
- Billberg, G.J. Enumeratio Insectorum in Museo Gust. Joh. Billberg; Typis Gadelianis: Stockholm, Sweden, 1820; p. 138. [Google Scholar]
- Schoenherr, C.J. Curculionides. [Tabula synoptica familiae Curculionidum]. Isis Oken 1823, 13, 1132–1146. [Google Scholar]
- Alonso-Zarazaga, M.A.; Wanat, M. 3.6.3. Apioninae Schoenherr, 1823. pp. 395–415. In Handbook of Zoology. Arthropoda: Insecta; Coleoptera, Beetles. Vol. 3. Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2014; p. 675. [Google Scholar]
- Alonso-Zarazaga, M.A.; Barrios, H.; Borovec, R.; Bouchard, P.; Caldara, R.; Colonnelli, E.; Gültekin, L.; Hlaváč, P.; Korotyaev, B.; Lyal, C.H.C.; et al. Cooperative catalogue of Palaearctic Coleoptera Curculionoidea. Monogr. Electrón. Soc. Entomol. Aragon. 2017, 8, 1–729. [Google Scholar]
- Friedman, A.L.L.; Freidberg, A. The Apionidae of Israel and the Sinai Peninsula (Coleoptera: Curculionoidea). Isr. J. Ent. 2007, 37, 55–180. [Google Scholar]
- Wencker, J.A. Apionides, tribu des curculionides. L’Abeille 1864, 1, 109–270. [Google Scholar]
- Desbrochers des Loges, J. Espèces inédites de curculionides de l’Ancien-Monde IV. [Cont.]. Le Frelon 1895, 4, 81–96. [Google Scholar]
- Gistel, J.N.F.X. Naturgeschichte des Thierreichs. Für Höhere Schulen Bearbeitet Durch Johannes Gistel. Mit Einem Atlas von 32 Tafeln (Darstellend 617 illuminirte Figuren) und Mehreren dem Texte Eingedruckten Xylographien; Hoffmann’sche Verlags-Buchhandlung: Stuttgart, Germany, 1848; p. 216. [Google Scholar]
- Alonso-Zarazaga, M.A. Revision of the supraspecific taxa in the Palaearctic Apionidae Schoenherr, 1823 (1. Introduction and subfamily Nanophyinae. Fragm. Ent. Roma 1989, 21, 205–262. [Google Scholar]
- Alonso-Zarazaga, M.A. 3.6.4. Nanophyinae Gistel, 1848. pp. 416–423. In Handbook of Zoology. Arthropoda: Insecta; Coleoptera, Beetles. Vol. 3. Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2014; p. 675. [Google Scholar]
- Des Gozis, M. Notes et remarques pour le futur catalogue de la faune gallo-rhénane (2e série). Rev. Ent. 1885, 4, 116–132. [Google Scholar]
- Reitter, E. Fauna Germanica. In Die Käfer des Deutsches Reiches. Nach der Analytische Methode Bearbeitet; V. Band; K. G. Lutz: Stuttgart, Germany, 1916; p. 343. [Google Scholar]
- Costa, A. Nuovi studii sulla entomologia della Calabria Ulteriore. Atti Della Accad. Sci. Fis. Nat. Napoli 1863, 1, 1–80. [Google Scholar]
- Tournier, H. De quelques nouveaux coléoptères d’Europe et d’Algérie. Ann. Soc. Ent. Fr. 1868, 7, 561–570. [Google Scholar]
- Giordani-Soika, A. Risultati scientifici delle spedizioni entomologiche di S.A.S. il Principe Alessandro della Torre e Tasso nel Bacino del Mediterraneo. I. Le specie mediterranee del genere Corimalia (Col. Curc.). Publ. Mus. Ent. Pietro Rossi 1937, 2, 1–29. [Google Scholar]
- Pic, M. Coléoptères nouveaux. Misc. Ent. 1897, 5, 26–29. [Google Scholar]
- Boheman, C.H. [new taxa]. In Genera et Species Curculionidum, cum Synonymia Hujus Familiae. Species Novae aut Hactenus Minus Cognitae, Descriptionibus a Dom. L. Gyllenhal, C. H. Boheman, O. J. Fahraeus et Entomologis Aliis Illustratae; Tomus octavus.—Pars secunda. Supplementum Continens; Parisiis: Roret; Schoenherr, C.J., Ed.; Flescher: Lipsiae, Germany, 1845; pp. 1–504. [Google Scholar]
- Gyllenhal, L. [new taxa]. In Genera et Species Curculionidum, cum Synonymia Hujus Familiae. Species Novae aut Hactenus Minus Cognitae, Descriptionibus a Dom. Leonardo Gyllenhal, C. H. Boheman, et Entomologis Aliis Illustratae; Tomus quartus.—Pars secunda; Parisiis: Roret; Schoenherr, C.J., Ed.; Flescher: Lipsiae, Germany, 1838; pp. 601–1124. [Google Scholar]
- Latreille, P.A. Histoire Naturelle, Générale et Particulière, des Crustacés et des Insectes. Ouvrage Faisant Suite aux Oeuvres de Leclerc de Buffon, et Partie du Cours Complet D’histoire Naturelle Rédigé Par C. S. Sonnini, Membre de Plusieurs Sociétés Savantes; Tome troisième; F. Dufart: Paris, France, 1802; p. 467. [Google Scholar]
- Thomson, C.G. Skandinaviens Coleoptera, Synoptiskt Bearbetade; Berlingska Bocktryckeriet: Lund, Sweden, 1859; p. 290. [Google Scholar]
- Caldara, R.; Franz, N.M.; Oberprieler, R.G. 3.7.10. Curculioninae Laterille, 1802. pp. 589–628. In Handbook of Zoology. Arthropoda: Insecta; Coleoptera, Beetles. Vol. 3. Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2014; p. 675. [Google Scholar]
- Sprick, P. Suitability of an insect group for the Habitats Directive of the EU: The weevil subfamily Bagoinae (Col., Curculionidae). Contributions to the Ecology of Phytophagous Beetles VII. Snudebiller 2001, 2, 7–40. [Google Scholar]
- Bodenheimer, F.S. Prodromus Faunae Palestinae. Essai sur les élements zoogéographiques et historiques du sud-ouest du sous-régne paléarctique. Mém. Inst. Egypte 1937, 33, 1–286. [Google Scholar]
- Germar, E.F. Miscellen und Correspondenz-Nachrichten. Mag. Ent. 1817, 2, 339–341. [Google Scholar]
- Dejean, P.F.M.A. Catalogue de la Collection de Coléoptères de M. le Baron Dejean; Crevot: Paris, France, 1821. [Google Scholar]
- Schilsky, J. Die Käfer Europa’s. Nach der Natur beschrieben von Dr. H.C. Kuster und Dr. G. Kraatz. Heft 44; Bauer und Raspe (E. Küster): Nürnberg, Germany, 1907; p. 100. [Google Scholar]
- Pic, M. Descriptions d’un Bryaxis et de plusieurs malacodermes ou rhyncophores. L’Échange Rev. Linn. 1904, 20, 49–51. [Google Scholar]
- Gyllenhal, L. [new taxa]. In Genera et Species Curculionidum, Cum Synonymia Hujus Familiae. Species Novae Aut Hactenus Minus Cognitae, Descriptionibus a Dom. Leonardo Gyllenhal, C. H. Boheman, et Entomologis Aliis Illustratae; Tomus tertius.—Pars secunda; Parisiis: Roret; Schönherr, C.J., Ed.; Flescher: Lipsiae, Germany, 1836; pp. 506–858. [Google Scholar]
- Dieckmann, L. Beiträge zur Insektenfauna der DDR: Coleoptera—Curculionidae (Tanymecinae, Leptopiinae, Cleoninae, Tanyrhynchinae, Cosonina, Raymondionyminae, Bagoinae, Tanysphyrinae). Beitr. Entomol. 1983, 33, 257–381. [Google Scholar]
- Cunev, J. Nové a zaujívané nálezy chrobákov (Coleoptera) nadčel`ade Curculionoidea na území Slovenska. Entomofauna Carpathica 2013, 25, 1–14. [Google Scholar]
- Blecher, M.; Blecher, I. Changes in vegetation in Nahal Boqeq: Is it due to the aqua pollution? Ecol. Vesviva 2011, 4, 255–256. (In Hebrew) [Google Scholar]
- Fåhraeus, O.I. [new taxa]. In Genera et Species Curculionidum, Cum Synonymia Hujus Familiae. Species Novae Aut Hactenus Minus Cognitae, Descriptionibus a Dom. Leonardo Gyllenhal, C. H. Boheman, O. J. Fåhraeus, et Entomologis Aliis Illustratae; Tomus octavus.—Pars secunda. Supplementum Continens; Parisiis: Roret; Schoenherr, C.J., Ed.; Flescher: Lipsiae, Germany, 1845; p. 504. [Google Scholar]
- Bina Perl, R.G.; Gafny, S.; Malka, Y.; Renan, S.; Woodhams, D.C.; Rollins-Smith, L.; Pask, J.D.; Bletz, M.C.; Geffen, E.; Vences, M. Natural history and conservation of the rediscovered Hula painted frog, Latonia nigriventer. Cont. Zool. 2017, 86, 11–37. [Google Scholar]
- Tournier, H. Matériaux pour servir à la monographie de la tribe des Erirrhinides de la familie des Curculionides (Coléoptères). Ann. Soc. Ent. Belg. 1874, 17, 63–116. [Google Scholar]
- Chevrolat, L.A.A. Description de coléoptères nouveaux d’Algérie. Rev. Mag. Zool. Pure Appl. 1860, 12, 509–511. [Google Scholar]
- Roesler, U. In Memoriam Dozent Dr. Stanislaw Blezsynski. Ent. Nachr. 1971, 18, 33–36. [Google Scholar]
- Reitter, E. Neue Coleopteren aus Europa, den angrenzenden Ländern und Sibirien, mit Bemerkungen über bekannte Arten. Neunter Teil. Deutsch. Ent. Zeit. 1890, 10, 145–164. [Google Scholar]
- Herbst, J.F.W. Natursystem Aller Bekannten in- und Ausländischen Insekten, Als Eine Fortsetzung der von Büffonschen Naturgeschichte; Der Käfer Sechster Theil; Joachim Pauli: Berlin, Germany, 1795; p. 520. [Google Scholar]
- Rosenhauer, W.G. Beiträge zur Insekten-Fauna Europas, Erstes Bändchen; Enthält die Beschreibung von Sechzig Neuen Käfern aus Bayern, Tyrol, Ungarn, etc., Sowie die Käfer Tyrols, Nach Dem Ergebnisse von Vier Reisen Zusammengestellt; Theodor Blaesing: Erlangen, Germany, 1847; p. 159. [Google Scholar]
- Schilsky, J. Die Käfer Europa’s. Nach der Natur Beschrieben von Dr. H.C. Küster und Dr. G. Kraatz. Heft 47; Bauer und Raspe (E. Küster): Nürnberg, Germany, 1911; p. 100. [Google Scholar]
- Schoenherr, C.J. Continuatio tabulae synopticae familiae curculionidum. Isis Oken 1825, 5, 581–588. [Google Scholar]
- Schoenherr, C.J. Genera et Species Curculionidum, Cum Synonymia Hujus Familiae. Species Novae aut Hactenus Minus Cognitae, Descriptionibus a Dom. L. Gyllenhal, C. H. Boheman, O. J. Fåhraeus et Entomologis Aliis Illustratae; Tomus septimus. Pars secunda. Supplementum Continens; Parisiis: Roret; Flescher: Lipsiae, Germany, 1843; p. 461. [Google Scholar]
- Caldara, R. A taxonomic revision of the weevil genus Picia Tournier, 1895 (Coleoptera: Curculionoidea: Erirhinidae). Zootaxa 2008, 1959, 39–57. [Google Scholar]
- Grachev, V.G. Family Curculionidae Latreille, 1802. pp. 196–198. In Plant-Arthropod Interactions in the Early Angiosperm History: Evidence from the Cretaceous of Israel; Part II. Fossil Insects in the Cretaceous Mangrove Facies of Southern Negev, Israel; pp. 189–224; Anisyutkin, L.N., Grachev, V.G., Ponomarenko, A.G., Rasnitsyn, A.P., Vršanský, P., Krassilov, V., Rasnitsyn, A., Eds.; Pensoft Publishers: Sofia, Bulgaria; Brill: Leiden, The Netherlands, 2008; p. 229. [Google Scholar]
- Krassilov, V.; Silantieva, N.; Lewy, Z. Part I. Traumas on Fossil Leaves from the Cretaceous of Israel, pp. 1–187. In Plant-Arthropod Interactions in the Early Angiosperm History: Evidence from the Cretaceous of Israel; Krassilov, V., Rasnitsyn, A., Eds.; Pensoft Publishers: Sofia, Bulgaria; Brill: Leiden, The Netherlands, 2008. [Google Scholar]
- Rabinovich, R.; Hanan, H.; Schudack, M.; Schudack, U.; Ashckenazi-Polivoda, S.; Rogolsky, G. A late Cretaceous elasmosaurid of the Tethys Sea margins (southern Negev, Israel), and its palaeogeographic reconstruction. Neth. J. Geosci. 2015, 94, 73–86. [Google Scholar]
- Alfieri, A. The Coleoptera of Egypt. Mém. Soc. Ent. Egypte 1976, 5, 1–362. [Google Scholar]
- Korotyaev, B.A. Family Curculionidae. pp. 145–149. In Key to Harmful and Useful Insects and Mites on Grain Plants in the USSR; Kopaneva, L.M., Ed.; Kolos: Leningrad, Russia, 1980. (In Russian) [Google Scholar]
- Marshall, G.A.K. On the West African species of Echinocnemus Schoen. (Col., Curculionidae). Ann. Mag. Nat. Hist. 1950, 3, 765–774. [Google Scholar] [CrossRef]
- Oberprieler, R. 3.7.1. Brachycerinae Billberg, 1820. pp. 424–451. In Handbook of Zoology. Arthropoda: Insecta; Coleoptera, Beetles. Vol. 3. Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2014. [Google Scholar]
- Singh, J.; Dhaliwal, G.S. Rice root weevil problem in India—An overview. Ind. J. Ent. 1990, 52, 217–222. [Google Scholar]
- Sahlberg, J.R. Coleoptera mediterranea orientalia, quae in Aegypto, Palaestina, Syria, Caramania atque in Anatolia occidentali anno 1904 collegerunt John Sahlberg et Unio Saalas. Öfversigt Fin. Vetensk-Soc. Förhandlingar (A) 1913, 55, 1–281. [Google Scholar]
- Schilsky, J. Die Käfer Europa’s. Nach der Natur Beschrieben von Dr. H.C. Kuster und Dr. G. Kraatz. Heft 44; Bauer und Raspe (E. Küster): Nürnberg, Germany, 1907; p. 100. [Google Scholar]
- Gyllenhal, L. [new taxa]. In Genera et Species Curculionidum, Cum Synonymia Hujus Familiae. Species Novae Aut Hactenus Minus Cognitae, Descriptionibus a Dom. Leonardo Gyllenhal, C. H. Boheman, et Entomologis Aliis Illustratae; Tomus tertius—Pars prima. [1836]; Parisiis: Roret; Schoenherr, C.J., Ed.; Flescher: Lipsiae, Germany, 1835; p. 505. [Google Scholar]
- Miller, L. Neue Käfer aus Kindermann’s Vorräthen. Wien. Ent. Monatsch. 1861, 5, 201–209. [Google Scholar]
- Caldara, R. Curculionidae: Curculioninae, pp. 117–172. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Brill: Leiden, The Netherlands, 2013; Volume 8, p. 700. [Google Scholar]
- Zumpt, F. Revision der Genera Notaris Germ., Lepidonotaris m., Thryogenes Bed., Grypus Germ., Icaris Tourn. und Picianus m. (Col. Curc.). Col. Cent. 1929, 3, 213–239. [Google Scholar]
- Faust, J. Die europäischen und asiatischen Arten der Gattungen Erirhinus, Notaris, Icaris, Dorytomus. Bull. Soc. Impériale Nat. Moscou 1882, 57, 113–188. [Google Scholar]
- Benedikt, S.; Borovec, R.; Fremuth, J.; Kratky, J.; Schoen, K.; Skuhrovec, J.; Tryzna, M. Annotated checklist of weevils (Coleoptera: Curculionoidea excepting Scolytinae and Platypodinae) of the Czech Republic and Slovakia. Klapalekiana 2010, 46, 1–363. [Google Scholar]
- Dieckmann, L. Beiträge zur Insektenfauna der DDR: Coleoptera—Curculionidae (Erirhinae). Beitr. Entomol. 1986, 36, 119–181. [Google Scholar]
- Tournier, H. [Rectification]. Bull. Soc. Ent. Fr. 1895, 63. [Google Scholar]
- David’yan, G.E. Interactive Agricultural Ecological Atlas of Russia and Neighboring Countries. Economic Plants and their Diseases, Pests and Weeds. Hydronomus sinuaticollis Faust—Rice Water Weevil. 2009. Available online: http://www.agroatlas.ru/en/content/pests/Hydronomus_sinuaticollis/index.html (accessed on 11 June 2018).
- Reitter, E. Coleopterologische Ergebnisse der im Jahre 1886 und 1887 in Transcaspien von Dr. G. Radde, Dr. A. Walter und A. Konchin ausgeführten Expedition. Verh. Naturforschenden Vereines Brünn 1889, 27, 95–133. [Google Scholar]
- Schoenherr, C.J. Curculionidum Dispositio Methodica Cum Generum Characteribus, Descriptionibus Atque Observationibus Variis, Seu Prodromus ad Synonymiae Insectorum, Partem IV; Fridericum Fleischer: Lipsiae, Germany, 1826; p. 338. [Google Scholar]
- Isaev, A.Y. Opredelitel Zhukov Srednego Povolzhia. Part 3. Polyphaga—Phytophaga; Vector-S: Ulyanovsk, Russia, 2007; p. 256. (In Russian) [Google Scholar]
- Boheman, C.H. [new taxa]. In Genera et Species Curculionidum, Cum Synonymia Hujus Familiae. Species Novae Aut Hactenus Minus Cognitae, Descriptionibus a Dom. Leonardo Gyllenhal, C. H. Boheman, et Entomologis Aliis Illustratae; Tomus tertius.—Pars secunda; Parisiis: Roret; Schoenherr, C.J., Ed.; Flescher: Lipsiae, Germany, 1836; Volume 4, 505p. [Google Scholar]
- Schoenherr, C.J. Genera et Species Curculionidum, Cum Synonymia Hujus Familiae. Species Novae Aut Hactenus Minus Cognitae, Descriptionibus a Dom. Leonardo Gyllenhal, C. H. Boheman et Entomologis Aliis Illustratae; Tomus tertius. Pars prima [1836]; Parisiis: Roret; Flescher: Lipsiae, Germany, 1835; p. 505. [Google Scholar]
- Caldara, R. Erirhinidae. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2011; Volume 7, pp. 192–198. [Google Scholar]
- Friedman, A.L.L. The first record of the Azolla frond weevil Stenopelmus rufinasus (Curculionidae: Brachycerinae: Tanysphyrini) in Israel. Isr. J. Ent. 2017, 47, 103–106. [Google Scholar]
- Hill, M.P.; McConnachie, A.J. Azolla filiculoides Lamarck (Azollaceae). In Biological Control of Tropical Weeds Using Arthropods; Muniappan, R., Reddy, G.V.P., Raman, A., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 74–87. [Google Scholar]
- Dufour-Dror, J.-M. Invasive Aquatic Plants. Kalanit. 2014. Available online: http://www.kalanit.org.il/?p=279 (accessed on 15 October 2017). (In Hebrew).
- Lacordaire, J.T. Histoire Naturelle des Insectes. Genera des Coléoptères ou Exposé Méthodique et Critique de tous les Genres Proposés Jusqu’ici Dans cet Ordre D’insectes; Tome Sixième; Roret: Paris, France, 1863; p. 637. [Google Scholar]
- Marshall, G.A.K. On the genus Aorus, Schh. (Coleoptera, Curculionidæ). Ann. Mag. Nat. Hist. 1919, 4, 338–343. [Google Scholar] [CrossRef]
- Brancsik, K. Coleoptera africana nova. Trencsén Természettud. Egylet Évkönyve 1989, 19–20, 108–131. [Google Scholar]
- Schoenherr, C.J. Genera et Species Curculionidum, Cum Synonymia Hujus Familiae. Species Novae Aut Hactenus Minus Cognitae, Descriptionibus a Dom. Leonardo Gyllenhal, C. H. Boheman et Entomologis Aliis Illustratae; Tomus primus.—Pars prima; Parisiis: Roret, Kenya, 1833; p. 381. [Google Scholar]
- Prena, J.; Colonnelli, E.; Hespenheid, H.A. 3.7.9. Conoderinae Schoenherr, 1823. pp. 577–589. In Handbook of Zoology. Arthropoda: Insecta; Coleoptera, Beetles. Vol. 3. Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2014; p. 675. [Google Scholar]
- Colonnelli, E. Catalogue of Ceutorhynchinae of the World, with a Key to Genera (Insecta: Coleoptera: Curculionidae); Argania Edito: Barcelona, Spain, 2004; p. 124. [Google Scholar]
- Korotyaev, B.A. A review of the weevil genus Rhinoncomimus Wagner (Coleoptera: Curculionidae: Ceutorhynchinae). Ent. Abh. 2006, 63, 99–122. [Google Scholar]
- Rheinheimer, J.; Hassler, M. Die Rüsselkäfer Baden-Württembergs; Verlag Regionalkultur: Ubstadt-Weiher, Germany, 2010; p. 944. [Google Scholar]
- Colonnelli, E. Checklist of Phytobiini of the world, with a key to the genera and description of a new species from South Africa (Coleoptera, Curculionidae, Ceutorhynchinae). Fragm. Ent. 1986, 19, 155–168. [Google Scholar]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Caracteribus, Differentiis, Synonymis; Laurentii Salvii: Holmiae, Sweden, 1758; p. 824. [Google Scholar]
- Reich, G.C. Mantissae Insectorum Iconibus Illustratae Species Novas Aut Nondum Depictas Exhibentis; Felsecker: Norimbergae, Germany, 1797; p. 16. [Google Scholar]
- Kirsch, T.F.W. Neue oder seltene Rüsselkäfer-Arten aus dem Gebiete des Mittelmeerbeckens. Ent. Monatsbl. 1881, 2, 3–16. [Google Scholar] [CrossRef]
- Caldara, R. Revisione delle specie paleartiche del genere Gymnetron (Insecta, Coleoptera: Curculionidae). Aldrovandia 2008, 4, 27–104. [Google Scholar]
- Desbrochers des Loges, J. Espèces inédites de curculionides de l’Ancien Monde. VI. Le Frelon 1900, 8, 1–16. [Google Scholar]
- Brisout de Barneville, C.N.F. Méthode dichotomique appliquée aux Tychius de France et description de quelques espèces nouvelles des genres Tychius et Miccotrogus. Ann. Soc. Ent. Fr. 1863, 2, 756–780. [Google Scholar]
- Caldara, R. Revisione tassonomica delle specie paleartiche del genere Tychius Germar (Coleoptera Curculionidae). Mem. Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 1990, 25, 51–218. [Google Scholar]
- Stephens, J.F. Illustrations of British Entomology; or, a Synopsis of Indigenous Insects: Containing Their Generic and Specific Distinctions; with an Account of Their Metamorphoses, Times of Appearance, Localities, Food, and Economy, as Far as Practicable. Mandibulata; Baldwin, Cradock: London, UK, 1831; p. 413. [Google Scholar]
- Marvaldi, A.E.; Lanteri, A.A.; Guadalupe del Rio, M.; Oberprieler, R.G. 3.7.5. Entiminae Schoenherr, 1823. pp. 529–570. In Handbook of Zoology. Arthropoda: Insecta; Coleoptera, Beetles. Vol. 3. Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2014. [Google Scholar]
- Velázquez de Castro, A.J.; Alonso Zarazaga, M.A.; Outerelo, R. Systematics of Sitonini (Col.: Curculionidae: Entiminae) with a hypothesis on evolution of feeding habits. Syst. Ent. 2007, 32, 212–331. [Google Scholar] [CrossRef]
- Velázquez de Castro, A.J.; Friedman, A.L.L.; Borovec, R. Sitonini (Curculionidae: Entiminae) of Israel. Isr. J. Ent. 2010, 40, 71–108. [Google Scholar]
- Fåhraeus, O.I. [new taxa]. In Genera et Species Curculionidum, Cum Synonymia Hujus Familiae; Species Novae Aut Hactenus Minus Cognitae, Descriptionibus a Dom. L. Gyllenhal, C. H. Boheman, O. J. Fåhraeus et Entomologis Aliis Illustratae; Tomus sextus. Pars prima; Parisiis: Roret; Schoenherr, C.J., Ed.; Flescher: Lipsiae, Germany, 1840; p. 474. [Google Scholar]
- Meregalli, M. 3.7.6. Lixinae Schoenherr, 1823. pp. 523–529. In Handbook of Zoology. Arthropoda: Insecta; Coleoptera, Beetles. Vol. 3. Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2014. [Google Scholar]
- Fabricius, J.C. Systema Eleutheratorum Secundum Ordines, Genera, Species: Adiectis Synonimis, Locis, Observationibus, Descriptionibus; Impensis Bibliopolii Academici Novi: Kiel, Germany, 1801; p. 687. [Google Scholar]
- Olivier, A.G. Entomologie, ou Histoire Naturelle des Insectes, Avec Leurs Caractères Génériques et Spécifiques, Leur Description, Leur Synonymie, et Leur Figure Enluminée. Coléoptères; Tome cinquième; Desray: Paris, France, 1807; p. 612. [Google Scholar]
- Scherf, H. Die Entwicklungsstadien der mitteleuropäischen Curculioniden (Morphologie, Bionomie, Ökologie) [Morphology, bionomy and ecology of developmental stages of Central European Curculionids]. Abh. Senckenberg. Nat. Ges. 1964, 506, 1–335. [Google Scholar]
- Volovnik, S.V. On the oviposition of weevils of the genus Lixus (Coleoptera, Curculionidae). Zool. Zhurnal 1994, 73, 49–54, (In Russian, Translated in Ent. Rev. 1995, 74, 115–120). [Google Scholar]
- Volovnik, S.V. On Distribution and Ecology of Some Species of Cleonines (Coleoptera, Curculionidae): IV. Genus Lixus F., Subgenus Eulixus Reitt. Entomol. Obozr. 2007, 86, 521–529, (In Russian, Translated in Ent. Rev. 2008, 87, 840–847). [Google Scholar] [CrossRef]
- Furth, D.G. Israel, a great biogeographic crossroads. Discovery 1975, 11, 2–13. [Google Scholar]
- Bytinski-Salz, H. The Ethiopian element in the insect fauna of Israel. In Proceedings of the XI International Congress of Entomology, Vienna, Austria, 17–25 August 1960. [Google Scholar]











© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedman, A.-L.-L. Review of the Hygrophilous Weevils in Israel (Coleoptera: Curculionoidea). Diversity 2018, 10, 77. https://doi.org/10.3390/d10030077
Friedman A-L-L. Review of the Hygrophilous Weevils in Israel (Coleoptera: Curculionoidea). Diversity. 2018; 10(3):77. https://doi.org/10.3390/d10030077
Chicago/Turabian StyleFriedman, Ariel-Leib-Leonid. 2018. "Review of the Hygrophilous Weevils in Israel (Coleoptera: Curculionoidea)" Diversity 10, no. 3: 77. https://doi.org/10.3390/d10030077




