Cross-Life Stage Effects of Aquatic Larval Density and Terrestrial Moisture on Growth and Corticosterone in the Spotted Salamander
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System
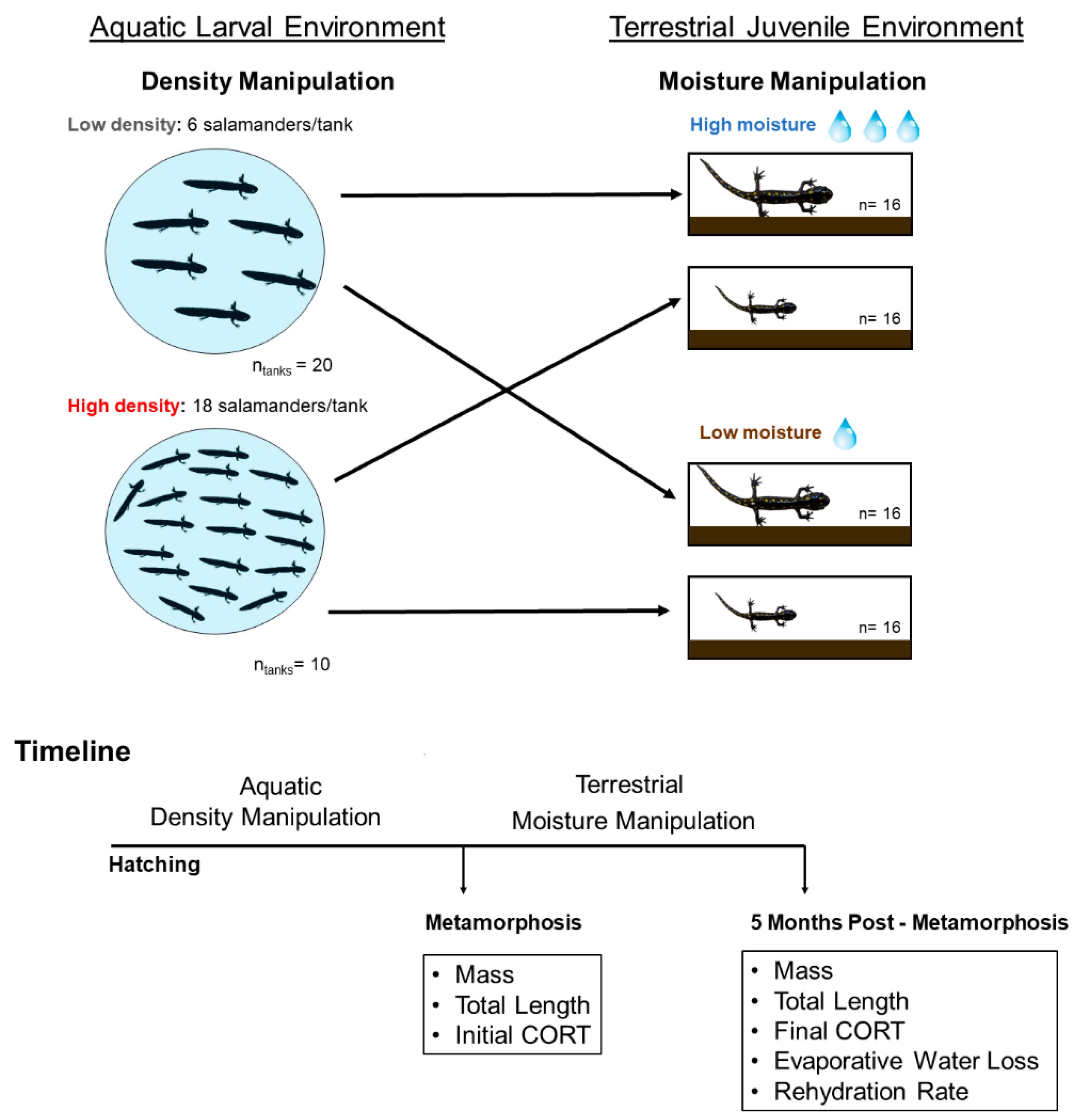
2.2. Larval Density Manipulation
2.3. Soil Moisture Manipulation
2.4. Dehydration and Rehydration Trials
2.5. Water-Borne Hormone Methods
2.6. Statistical Analyses
3. Results
3.1. Larval Density Manipulation
3.2. Moisture, Relative Humidity and Temperature in Terrariums
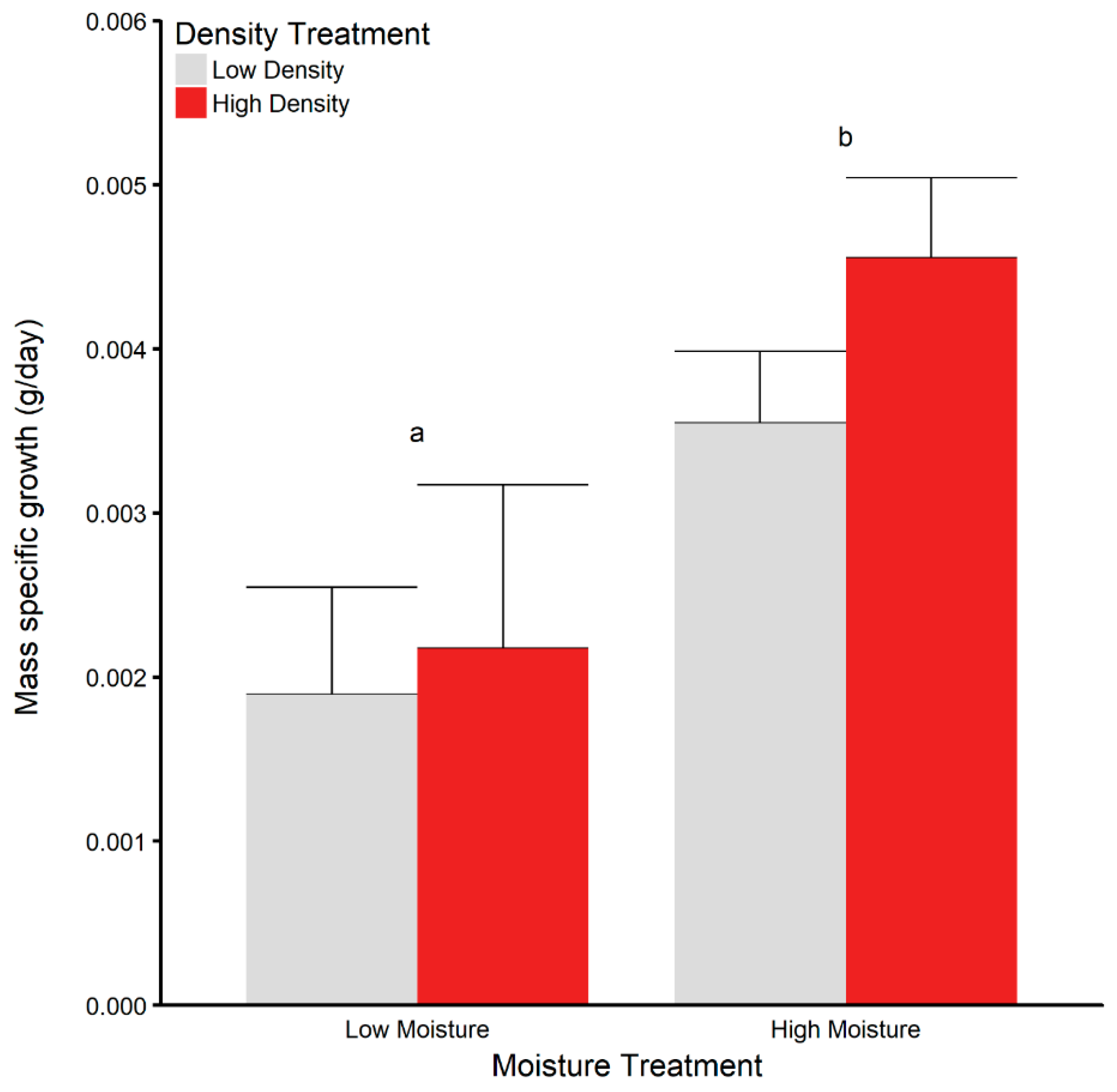
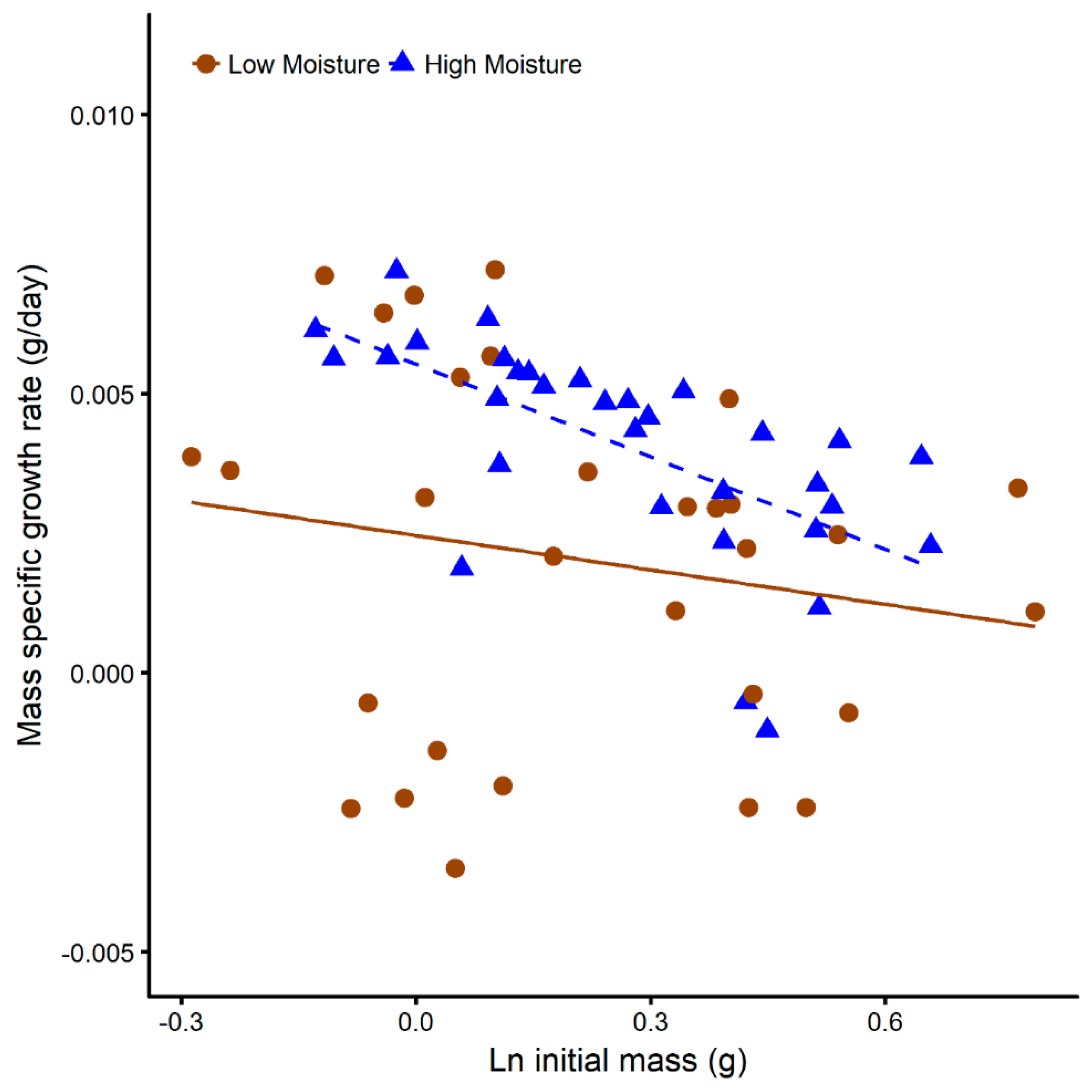
3.3. Juvenile Survival and Growth in Terrariums
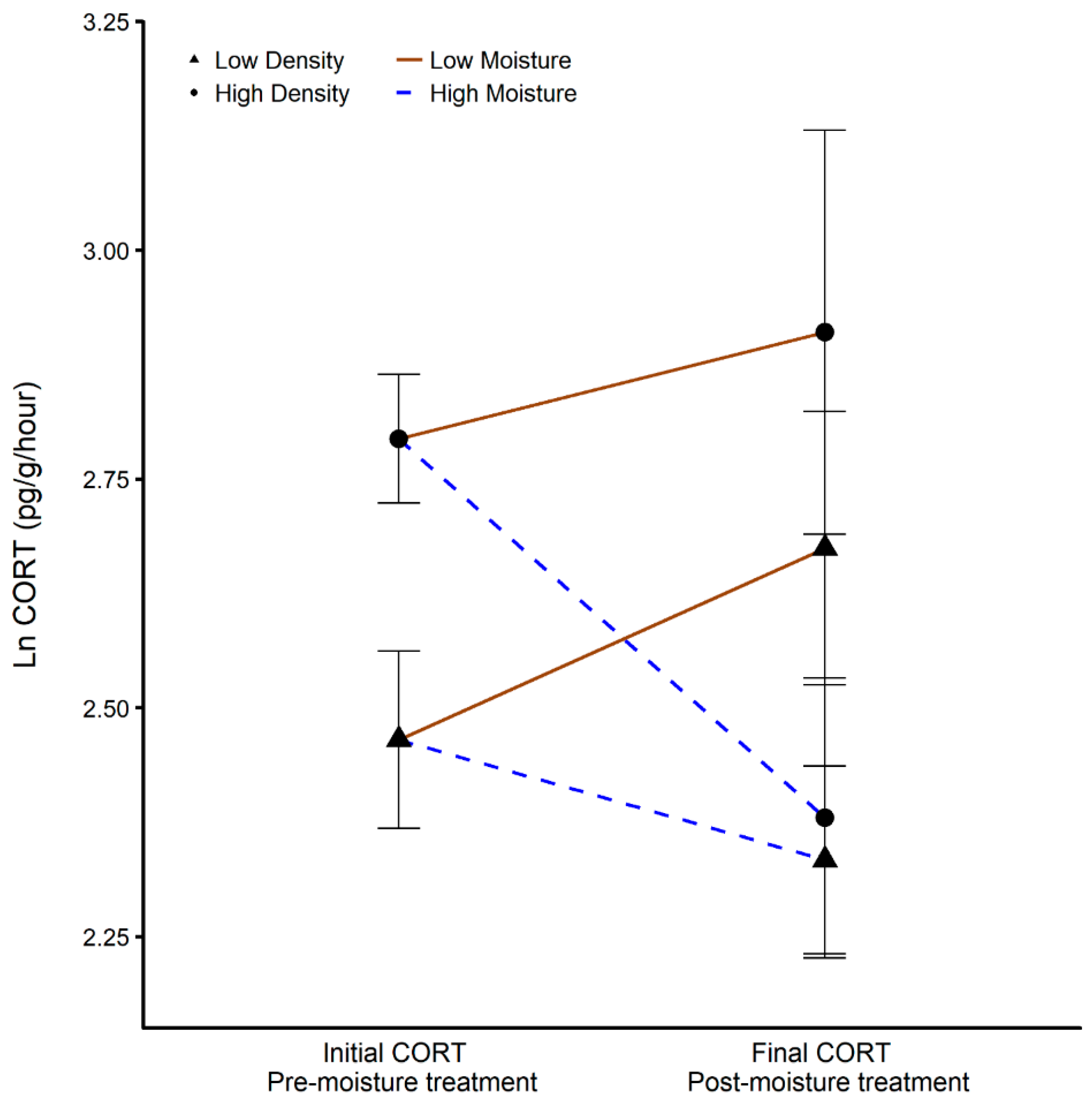
3.4. Corticostrone Release Rate
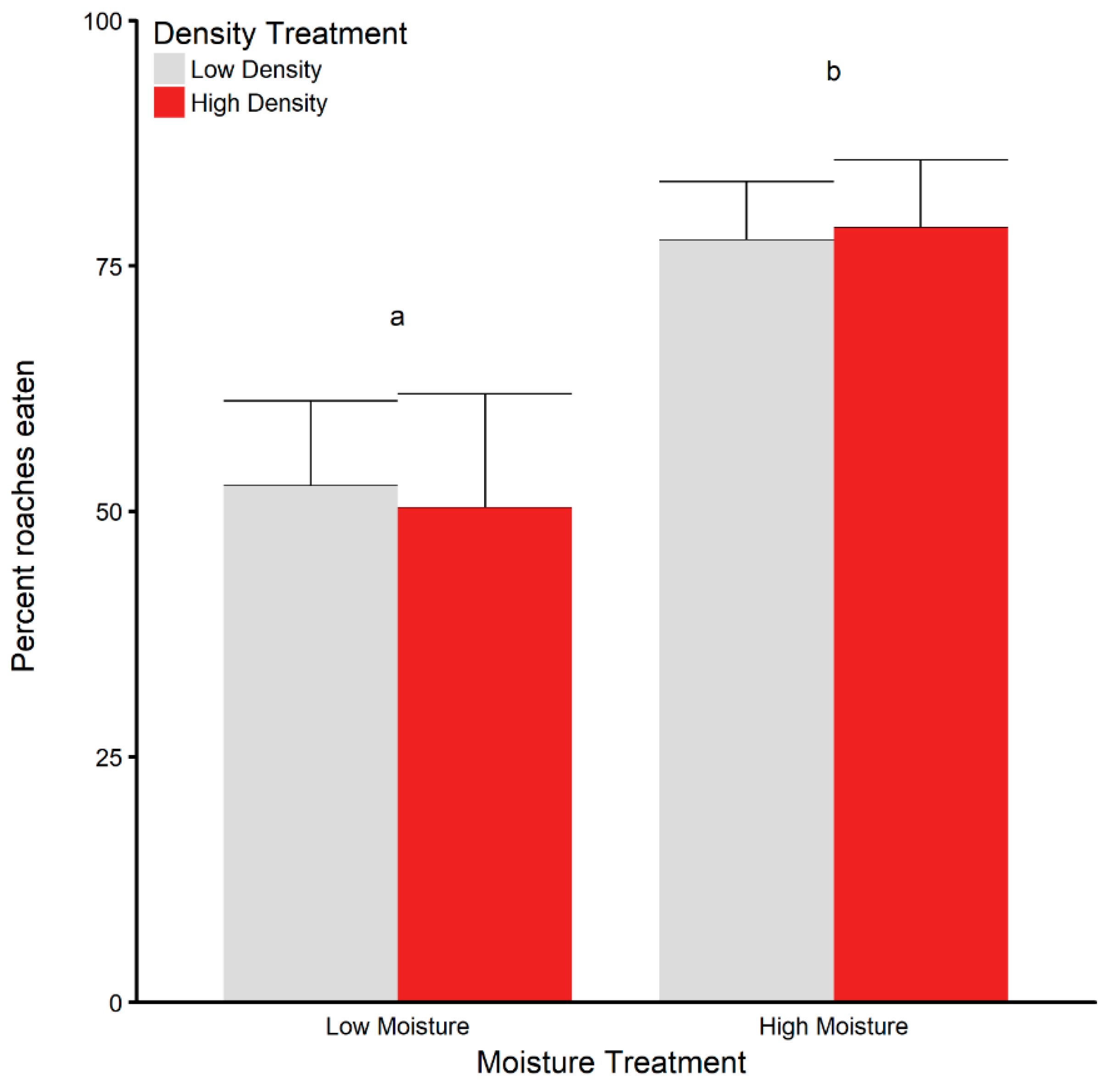
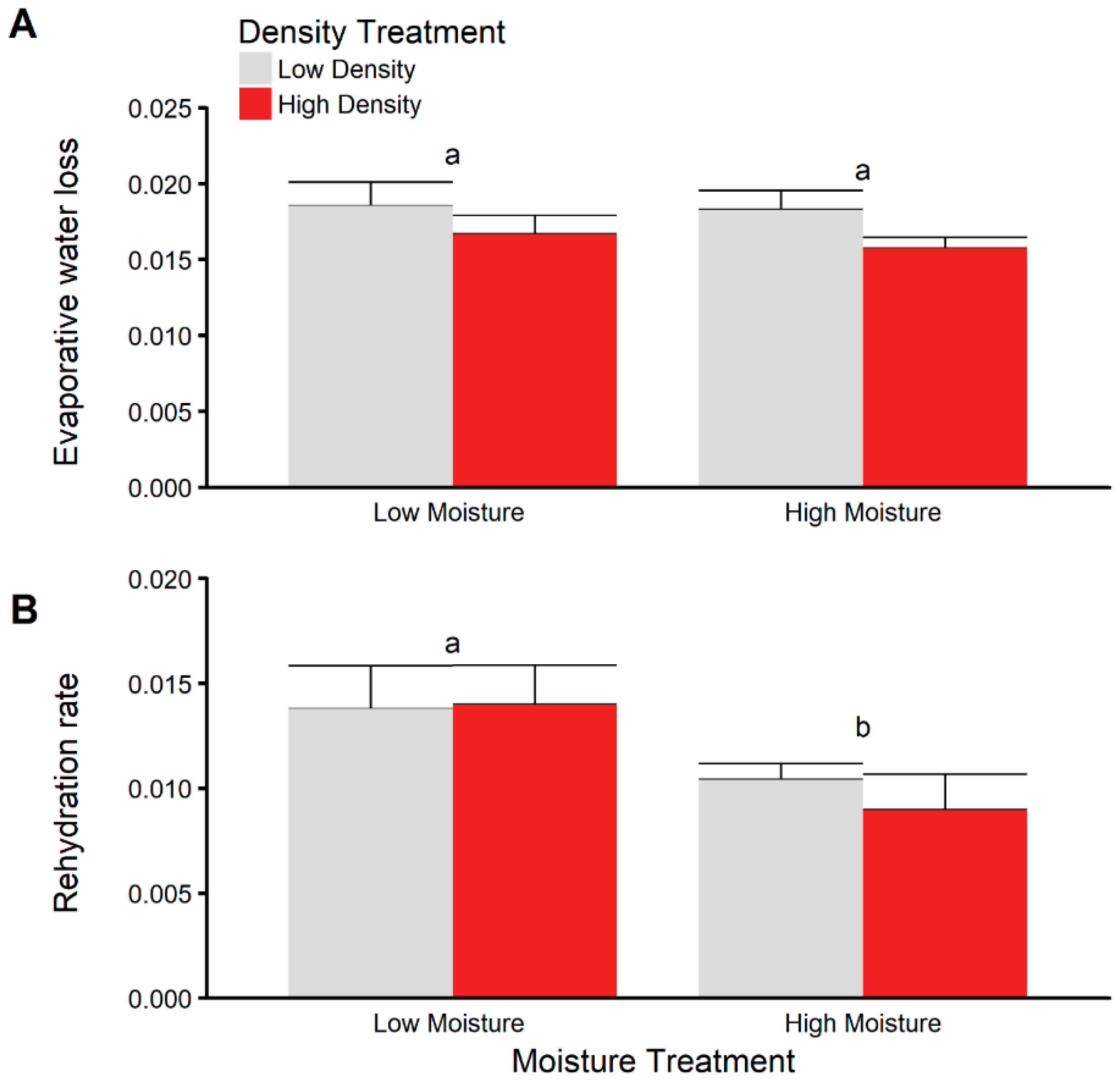
3.5. Dehydration and Rehydration Trials
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moran, N.A. Adaptation and constraint in the complex life cycles of animals. Annu. Rev. Ecol. Syst. 1994, 25, 573–600. [Google Scholar] [CrossRef]
- Ebenman, B. Evolution in organisms that change their niches during the life cycle. Am. Nat. 1992, 139, 990–1021. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Fox, C.W. The adaptive signficance of maternal effects. Trends Ecol. Evol. 1998, 13, 403–407. [Google Scholar] [CrossRef]
- Podolsky, R.; Moran, A. Integrating function across marine life cycles. Integr. Comp. Biol. 2006, 46, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pechenik, J. Larval experience and latent effects—Metamorphosis is not a new beginning. Integr. Comp. Biol. 2006, 46, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.J.; Morgan, S.G. Ecological and evolutionary consequences of linked life-history stages in the sea. Curr. Biol. 2011, 21, R718–R725. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mestre, I.; Saccoccio, V.L.; Iijima, T.; Collins, E.M.; Rosenthal, G.G.; Warkentin, K.M. The shape of things to come: Linking developmental plasticity to post-metamorphic morphology in anurans. J. Evol. Biol. 2010, 23, 1364–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, R.M.; Marshall, D.J. Phenotypic links among life-history stages are complex and context-dependent in a marine invertebrate: Interactions among offspring size, larval nutrition and postmetamorphic density. Funct. Ecol. 2013, 27, 1358–1366. [Google Scholar] [CrossRef]
- Auer, S.K. Phenotypic plasticity in adult life-history strategies compensates for a poor start in life in Trinidadian guppies (Poecilia reticulata). Am. Nat. 2010, 176, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.; Shine, R. Silver spoons and snake body sizes: Prey availability early in life influences long-term growth rates of free ranging pythons. J. Anim. Ecol. 2000, 69, 952–959. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Monaghan, P. Compensation for a bad start: Grow now, pay later? Trends Ecol. Evol. 2001, 16, 254–260. [Google Scholar] [CrossRef]
- Lindström, J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999, 14, 343–348. [Google Scholar] [CrossRef]
- Crespi, E.J.; Warne, R.W. Environmental conditions experienced during the tadpole stage alter post-metamorphic glucocorticoid response to stress in an amphibian. Integr. Comp. Biol. 2013, 53, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Raffel, T.R.; Halstead, N.T.; McMahon, T.A.; Johnson, S.A.; Boughton, R.K.; Martin, L.B. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131502. [Google Scholar] [CrossRef]
- Smith-Gill, S.J.; Berven, K.A. Predicting amphibian metamorphosis. Am. Nat. 1979, 113, 563–585. [Google Scholar] [CrossRef]
- Leips, J.; Travis, J. Metamorphic responses to changing food levels in two species of Hylid frogs. Ecology 1994, 75, 1345–1356. [Google Scholar] [CrossRef]
- Wilbur, H.M.; Collins, J.P. Ecological aspects of amphibian metamorphosis. Science 1973, 182, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.K. Experimental venue and estimation of interaction strength. Ecology 2002, 83, 2097–2101. [Google Scholar] [CrossRef]
- Brockelman, W.Y. An analysis of density effects and predation in Bufo americanus tadpoles. Ecology 1969, 50, 632–644. [Google Scholar] [CrossRef]
- Wilbur, H.M. Density-dependent aspects of growth and metamorphosis in Bufo americanus. Ecology 1977, 58, 196–200. [Google Scholar] [CrossRef]
- Scott, D.E.; Casey, E.D.; Donovan, M.F.; Lynch, T.K. Amphibian lipid levels at metamorphosis correlate to post-metamorphic terrestrial survival. Oecologia 2007, 153, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, J.F.; Vonesh, J.R. Consequences of life history switch point plasticity for juvenile morphology and locomotion in the Túngara frog. PeerJ 2015, 3, e1268. [Google Scholar] [CrossRef] [PubMed]
- Spight, T. The water economy of salamanders: Evaporative water loss. Physiol. Zool. 1968, 41, 195–203. [Google Scholar] [CrossRef]
- Cabrera-Guzmán, E.; Crossland, M.R.; Brown, G.P.; Shine, R. Larger body size at metamorphosis enhances survival, growth and performance of young cane toads (Rhinella marina). PLoS ONE 2013, 8, e70121. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, K. Water balance in estivating sirenid salamanders (Siren lacertina). Herpetologica 1990, 46, 400–406. [Google Scholar]
- Jørgensen, C.B. 200 years of amphibian water economy: From Robert Townson to the present. Biol. Rev. Camb. Philos. Soc. 1997, 72, 153–237. [Google Scholar] [CrossRef] [PubMed]
- Spotila, J.R. Role of temperature and water in the ecology of lungless salamanders. Ecol. Monogr. 1972, 42, 95–125. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Todd, B.D.; Blomquist, S.M.; Calhoun, A.J.K.; Gibbons, J.W.; Gibbs, J.P.; Graeter, G.J.; Harper, E.B.; Hocking, D.J.; Hunter, M.L.; et al. Effects of timber harvest on amphibian populations: Understanding mechanisms from forest experiments. Bioscience 2009, 59, 853–862. [Google Scholar] [CrossRef]
- Rothermel, B.B.; Semlitsch, R.D. An experimental investigation of landscape resistance of forest versus old-field habitats to emigrating juvenile amphibians. Conserv. Biol. 2002, 16, 1324–1332. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. Neuroendocrine-immune system interactions in amphibians: Implications for understanding global amphibian declines. Immunol. Res. 2001, 23, 273–280. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.; Matlina, E.; Maor, G.I.; Rosen, H.; McEwen, B.S. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992, 595, 195–200. [Google Scholar] [CrossRef]
- Welberg, L.A.M.; Seckl, J.R.; Holmes, M.C. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: Possible implications for behaviour. Neuroscience 2001, 104, 71–79. [Google Scholar] [CrossRef]
- Kirschman, L.J.; McCue, M.D.; Boyles, J.G.; Warne, R.W. Exogenous stress hormones alter energetic and nutrient costs of development and metamorphosis. J. Exp. Biol. 2017, 220, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Glennemeier, K.A.; Denver, R.J. Role for corticoids in mediating the response of Rana pipiens tadpoles to intraspecific competition. J. Exp. Zool. 2002, 292, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M.; Wikelski, M. Diurnal and nocturnal differences in hypothalamic-pituitary-adrenal axis function in Galápagos marine iguanas. Gen. Comp. Endocrinol. 2006, 145, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Cree, A.; Tyrrell, C.L.; Preest, M.R.; Thorburn, D.; Guillette, L.J. Protecting embryos from stress: Corticosterone effects and the corticosterone response to capture and confinement during pregnancy in a live-bearing lizard (Hoplodactylus maculatus). Gen. Comp. Endocrinol. 2003, 134, 316–329. [Google Scholar] [CrossRef]
- Woodley, S.K.; Painter, D.L.; Moore, M.C.; Wikelski, M.; Michael Romero, L. Effect of tidal cycle and food intake on the baseline plasma corticosterone rhythm in intertidally foraging marine iguanas. Gen. Comp. Endocrinol. 2003, 132, 216–222. [Google Scholar] [CrossRef]
- Crespi, E.J.; Denver, R.J. Roles of stress hormones in food intake regulation in anuran amphibians throughout the life cycle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Middlemis Maher, J.; Werner, E.E.; Denver, R.J. Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123075. [Google Scholar] [CrossRef] [PubMed]
- Narayan, E.J.; Cockrem, J.F.; Hero, J.M. Sight of a predator induces a corticosterone stress response and generates fear in an amphibian. PLoS ONE 2013, 8, e73564. [Google Scholar] [CrossRef] [PubMed]
- Creel, S.; Dantzer, B.; Goymann, W.; Rubenstein, D.R. The ecology of stress: Effects of the social environment. Funct. Ecol. 2013, 27, 66–80. [Google Scholar] [CrossRef]
- Davis, A.K.; Maerz, J.C. Assessing stress levels of captive-reared amphibians with hematological data: Implications for conservation initiatives. J. Herpetol. 2011, 45, 40–44. [Google Scholar] [CrossRef]
- Romero, L.M.; Wikelski, M. Exposure to tourism reduces stress-induced corticosterone levels in Galápagos marine iguanas. Biol. Conserv. 2002, 108, 371–374. [Google Scholar] [CrossRef]
- Gabor, C.R.; Davis, D.R.; Kim, D.S.; Zabierek, K.C.; Bendik, N.F. Urbanization is associated with elevated corticosterone in Jollyville Plateau salamanders. Ecol. Indic. 2018, 85, 229–235. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). IPCC Fifth Assessment Synthesis Report-Climate Change 2014 Synthesis Report; IPCC: Geneva, Switzerland, 2014; 167p. [Google Scholar]
- Greenberg, C.H.; Goodrick, S.; Austin, J.D.; Parresol, B.R. Hydroregime prediction models for ephemeral groundwater-driven sinkhole wetlands: A planning tool for climate change and amphibian conservation. Wetlands 2015, 35, 899–911. [Google Scholar] [CrossRef]
- Dupoué, A.; Angelier, F.; Lourdais, O.; Bonnet, X.; Brischoux, F. Effect of water deprivation on baseline and stress-induced corticosterone levels in the Children’s python (Antaresia childreni). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 168, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Whitford, W.G.; Vinegar, A. Homing, survivorship, and overwintering of larvae in spotted salamanders, Ambystoma maculatum. Copeia 1966, 1966, 515–519. [Google Scholar] [CrossRef]
- Petranka, J.W.; Rushlow, A.W.; Hopey, M.E. Predation by tadpoles of Rana sylvatica on embryos of Ambystoma maculatum: Implications of ecological role reversals by Rana (Predator) and Ambystoma (Prey). Herpetologica 1998, 54, 1–13. [Google Scholar]
- Brodman, R. Annual variation in the breeding success of two syntopic species of Ambystoma salamanders. J. Herpetol. 1995, 29, 111–113. [Google Scholar] [CrossRef]
- Wright, A.H.; Allen, A. The early breeding habits of Amblystoma punctatum. Am. Nat. 1909, 43, 687–692. [Google Scholar] [CrossRef]
- Madison, D.M. The emigration of radio-implanted spotted salamanders, Ambystoma maculatum. J. Herpetol. 1997, 31, 542–551. [Google Scholar] [CrossRef]
- Faccio, S.D. Postbreeding emigration and habitat use by Jefferson and spotted salamanders in Vermont. J. Herpetol. 2003, 37, 479–489. [Google Scholar] [CrossRef]
- Metts, B.S.; Hopkins, W.A.; Nestor, J.P. Interaction of an insecticide with larval density in pond-breeding salamanders (Ambystoma). Freshw. Biol. 2005, 50, 685–696. [Google Scholar] [CrossRef]
- Boone, M.; James, S. Interactions of an insecticide, herbicide, and natural stressors in amphibian community mesocosms. Ecol. Appl. 2003, 13, 829–841. [Google Scholar] [CrossRef]
- Figiel, C.R.; Semlitsch, R.D. Population variation in survival and metamorphosis of larval salamanders (Ambystoma maculatum) in the presence and absence of fish predation. Copeia 1990, 1990, 818–826. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabor, C.R.; Zabierek, K.C.; Kim, D.S.; da Barbiano, L.A.; Mondelli, M.J.; Bendik, N.F.; Davis, D.R. A non-invasive water-borne assay of stress hormones in aquatic salamanders. Copeia 2016, 104, 172–181. [Google Scholar] [CrossRef]
- Dunn, J.; Scheving, L.; Millet, P. Circadian variation in stress-evoked increases in plasma corticosterone. Am. J. Physiol. 1972, 223, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; James, J.D.; Stewart, C.; Scott, A.P. A non-invasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. J. Fish Biol. 2004, 65, 1233–1252. [Google Scholar] [CrossRef]
- Winters, A.; Gifford, M.E. Geographic variation in the water economy of a lungless salamander. Herpetol. Conserv. Biol. 2013, 8, 741–747. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Vienna: RFoundation for Statistical Computing 2016. Available online: https://www.R-project.org/ (accessed on 17 July 2018).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models; R Package: 2016. Available online: httpps://CRAN.R-project.org/package=nlme (accessed on 17 July 2018).
- Sinervo, B.; Adolph, S.C. Thermal sensitivity of growth rate in hatchling Sceloporus lizards: Environmental, behavioral and genetic aspects. Oecologia 1989, 78, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Hou, P.C.L. Cutaneous resistance to evaporative water loss in Taiwanese arboreal Rhacophorid frogs. Zool. Stud. 2012, 51, 988–995. [Google Scholar]
- Whitford, W.G.; Hutchison, V.H. Body size and metabolic rate in salamanders. Physiol. Zool. 1967, 40, 127–133. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Welsch, R.E. The hat matrix in regression and ANOVA. Am. Stat. 1978, 32, 17–22. [Google Scholar] [CrossRef]
- Fox, J. Applied Regression Analysis, Linear Models, and Related Methods; Sage Publications: Newbury Park, CA, USA, 1997; ISBN 080394540X. [Google Scholar]
- Earl, J.E.; Whiteman, H.H. Are commonly used fitness predictors accurate? A meta-analysis of amphibian size and age at metamorphosis. Copeia 2015, 103, 297–309. [Google Scholar] [CrossRef]
- Wells, K. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Skelly, D.K.; Kiesecker, J.M. Venue and outcome in ecological experiments: Manipulations of larval anurans. Oikos 2001, 94, 198–208. [Google Scholar] [CrossRef]
- Boone, M.D. Juvenile frogs compensate for small metamorph size with terrestrial growth: Overcoming the effects of larval density and insecticide exposure. J. Herpetol. 2005, 39, 416–423. [Google Scholar] [CrossRef]
- Goater, C.P. Growth and survival of postmetamorphic toads: Interactions among larval history, density, and parasitism. Ecology 1994, 75, 2264–2274. [Google Scholar] [CrossRef]
- Woodley, S.K. Life in the slow lane: Stress responses in Plethodontid salamanders. Herpetologica 2017, 73, 259–268. [Google Scholar] [CrossRef]
- Morey, S.; Reznick, D. Effects of larval density on postmetamorphic spadefoot toads (Spea hammondii). Ecology 2001, 82, 510–522. [Google Scholar] [CrossRef]
- Benard, M.F.; Middlemis Maher, J. Consequences of intraspecific niche variation: Phenotypic similarity increases competition among recently metamorphosed frogs. Oecologia 2011, 166, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Londos, P.L. Hydric constraints upon foraging in a terrestrial salamander, Desmognathus ochrophaeus (Amphibia: Plethodontidae). Oecologia 1984, 64, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Palmer, B.D. Climate change, multiple stressors, and the decline of ectotherms. Conserv. Biol. 2013, 27, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mestre, I.; Tejedo, M. Adaptation or exaptation? An experimental test of hypotheses on the origin of salinity tolerance in Bufo calamita. J. Evol. Biol. 2005, 18, 847–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudin-Bitterli, T.S.; Evans, J.P.; Mitchell, N.J. Geographic variation in adult and embryonic desiccation tolerance in a terrestrial-breeding frog. bioRxiv 2018. [Google Scholar] [CrossRef]
- Riddell, E.A.; Sears, M.W. Geographic variation of resistance to water loss within two species of lungless salamanders: Implications for activity. Ecosphere 2015, 6, 1–16. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charbonnier, J.F.; Pearlmutter, J.; Vonesh, J.R.; Gabor, C.R.; Forsburg, Z.R.; Grayson, K.L. Cross-Life Stage Effects of Aquatic Larval Density and Terrestrial Moisture on Growth and Corticosterone in the Spotted Salamander. Diversity 2018, 10, 68. https://doi.org/10.3390/d10030068
Charbonnier JF, Pearlmutter J, Vonesh JR, Gabor CR, Forsburg ZR, Grayson KL. Cross-Life Stage Effects of Aquatic Larval Density and Terrestrial Moisture on Growth and Corticosterone in the Spotted Salamander. Diversity. 2018; 10(3):68. https://doi.org/10.3390/d10030068
Chicago/Turabian StyleCharbonnier, Julie F., Jacquelyn Pearlmutter, James R. Vonesh, Caitlin R. Gabor, Zachery R. Forsburg, and Kristine L. Grayson. 2018. "Cross-Life Stage Effects of Aquatic Larval Density and Terrestrial Moisture on Growth and Corticosterone in the Spotted Salamander" Diversity 10, no. 3: 68. https://doi.org/10.3390/d10030068




