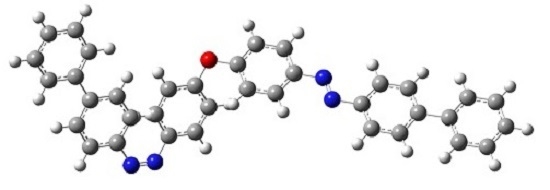
4,4′-Bis[4-(4′-hydroxyphenyl)phenylazo]diphenyl Ether
Abstract
:1. Introduction
2. Results
3. Discussion
4. Experimental Section
4.1. General Information
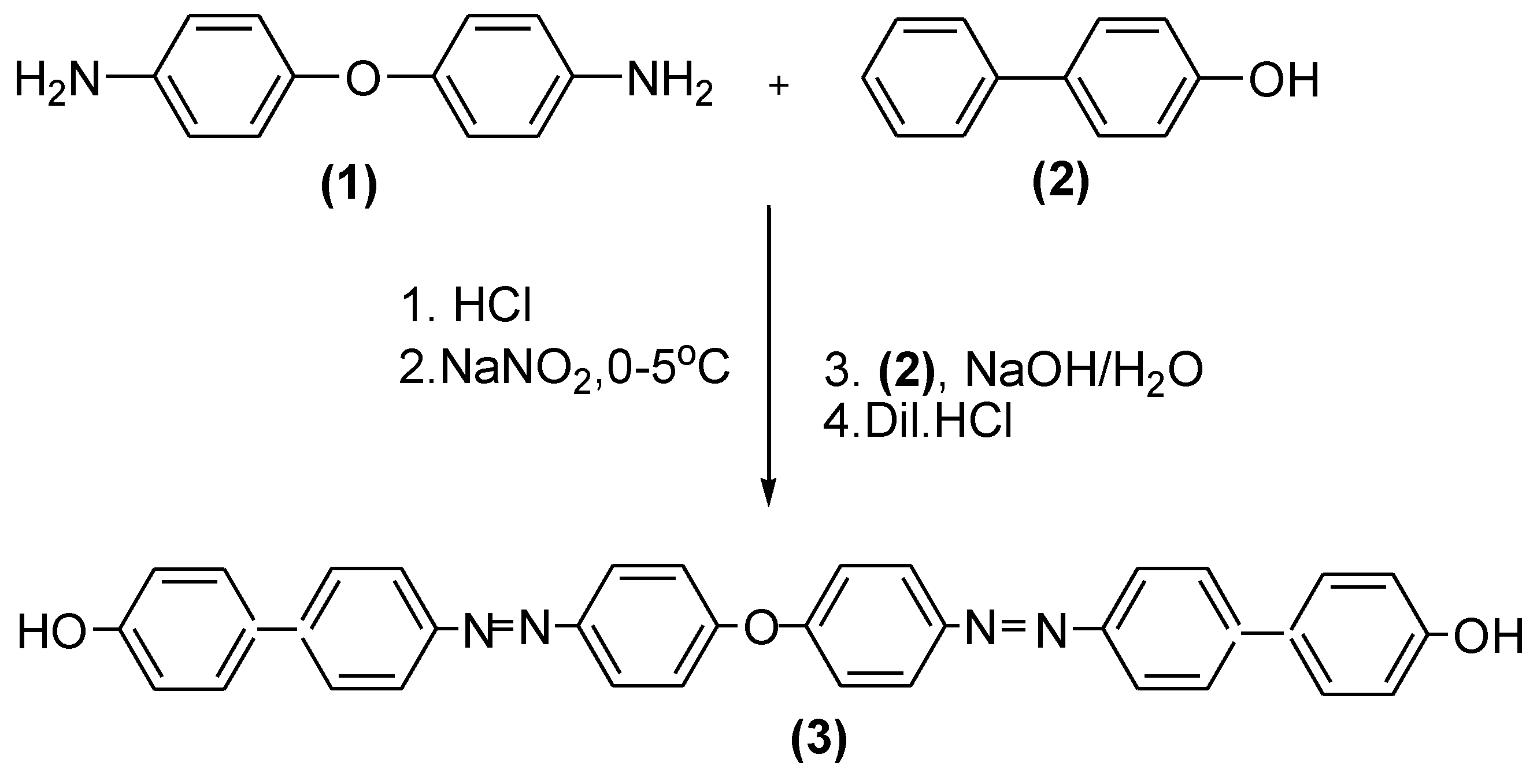
4.2. Synthesis of 4,4′-Bis[4-(4′-hydroxyphenyl)phenylazo]diphenyl Ether(3)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bandara, H.M.D.; Burdette, S.C. Photo-isomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Meng, X.Z.; Jiang, H.B.; Zhou, T.Y.; Gong, C.B.; Fu, X.; Shi, S.Q.K. Synthesis and characterization of photo and pH-responsive nanoparticles containing amino-substituted azobenzene. J. Mater. Chem. 2010, 20, 9133–9139. [Google Scholar] [CrossRef]
- Rau, H.; Lueddecke, E. On the rotation-inversion controversy on photoisomerization of azobenzenes, experimental proof of inversion. J. Am. Chem. Soc. 1982, 104, 1616–1620. [Google Scholar] [CrossRef]
- Kumar, G.S.; Neckers, D.C. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; Wiley-VCH: Weinheim, Germany, 2003; pp. 13–33. [Google Scholar]
- Zollinger, H. Color Chemistry: Syntheses, Properties and Applications of Organic Dyes and Pigments; VCH: New York, NY, USA, 1987; pp. 85–87. [Google Scholar]
- Gordon, P.F.; Gregory, P. Organic Chemistry in Color; Springer: New York, NY, USA, 1983; pp. 95–105. [Google Scholar]
- Stevenson, D.D. Tartrazine, Azo, and Non-Azo Dyes. In Food Allergy: Adverse Reactions to Foods and Food Additives; John Wiley & Sons Ltd.: Chichester, UK, 2014; pp. 384–392. [Google Scholar]
- Cheng, X.; Li, Q.; Li, C.; Qin, J.; Li, Z. Azobenzene-Based Colorimetric Chemosensors for Rapid Naked-Eye Detection of Mercury (II). Chem. Eur. J. 2011, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
- Merino, E. Synthesis of azobenzene: colored pieces of molecular material chem. Soc. Rev. 2011, 40, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J. Rational selection of oral 5-aminosalicylate formulations and prodrugs for the treatment of ulcerative colitis. Am. J. Gastroenterol. 2002, 97, 2939–2942. [Google Scholar] [CrossRef] [PubMed]
- Morihiro, K.; Hasegawa, O.; Mori, S.; Tsunoda, S.; Morihiro, K. C5-azobenzene-functionalized locked nucleic acid uridine: isomerization properties, hybridization ability, and enzymatic stability. Org. Biomol. Chem. 2015, 13, 5209–5214. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chen, S.; Guo, X.; Zhang, Y.; Zhang, H. Azobenzene-containing molecularly imprinted polymer microspheres with photo-and thermoresponsive template binding properties in pure aqueous media by atom transfer radical polymerization. Langmuir 2012, 28, 9767–9777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Zhang, M.; Moers, C.; Chen, S.L.; Xu, H.P.; Wang, Z.Q.; Zhang, X.; Li, Z.B. Block copolymer aggregates with photo-responsive switches: towards a controllable supramolecular container. Polymer. 2009, 50, 4821–4828. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef] [PubMed]
- Itoga, K.; Yamato, M.; Kobayashi, J.; Kikuchi, A.; Okano, T. Cell micro patterning using photopolymerization with a liquid crystal device commercial projector. Biomaterials 2004, 25, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Jaunet-Lahary, T.; Chantzis, A.; Chen, K.J. Designing Efficient Azobenzene and Azothiophene Nonlinear Optical Photochromes. J. Phys. Chem. C. 2014, 49, 28831–28841. [Google Scholar] [CrossRef]
- Browne, W.R.; Feringa, B.L. Making molecular machines work. Nat. Nanotechnol. 2006, 1, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Li, Y.; Wang, C.T.; Jau, H.C.; Chen, C.W. Red, Green and Blue Reflections Enabled in an Optically Tunable Self-Organized 3D Cubic Nanostructured Thin Film. Adv. Mater. 2013, 25, 5050–5054. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bihari, B.; Filler, R.; Mandal, B.K. New azobenzene non-linear optical materials for eye and sensor protection. Opt. Mater. 2009, 32, 147–153. [Google Scholar] [CrossRef]
- Graydon, O. Photoisomerizaton: Molecular motors driven by light. Nat. Photon. 2015, 9. [Google Scholar] [CrossRef]
- Ivanka, S.; Zollinger, H. Azo coupling reactions structures and mechanisms. In Preparative Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 1983; pp. 1–66. [Google Scholar]
- Feringa, B.L.; Wesley, R.B. Molecular Switches; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopic, 3rd ed.; Thomson Learning Inc.: New York, NY, USA, 2001. [Google Scholar]
- Castellano, S.; Bothner-By, A.A. Analysis of NMR spectra by least squares. J. Chem. Phys. 1964, 41, 3863–3869. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, R.; Gul, A.; Akhter, Z. 4,4′-Bis[4-(4′-hydroxyphenyl)phenylazo]diphenyl Ether. Molbank 2016, 2016, M914. https://doi.org/10.3390/M914
Rahman R, Gul A, Akhter Z. 4,4′-Bis[4-(4′-hydroxyphenyl)phenylazo]diphenyl Ether. Molbank. 2016; 2016(4):M914. https://doi.org/10.3390/M914
Chicago/Turabian StyleRahman, Rahima, Asghari Gul, and Zareen Akhter. 2016. "4,4′-Bis[4-(4′-hydroxyphenyl)phenylazo]diphenyl Ether" Molbank 2016, no. 4: M914. https://doi.org/10.3390/M914