1,3-Butanediol Dibenzoate
Abstract
:1. Introduction
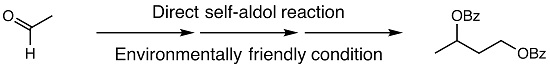
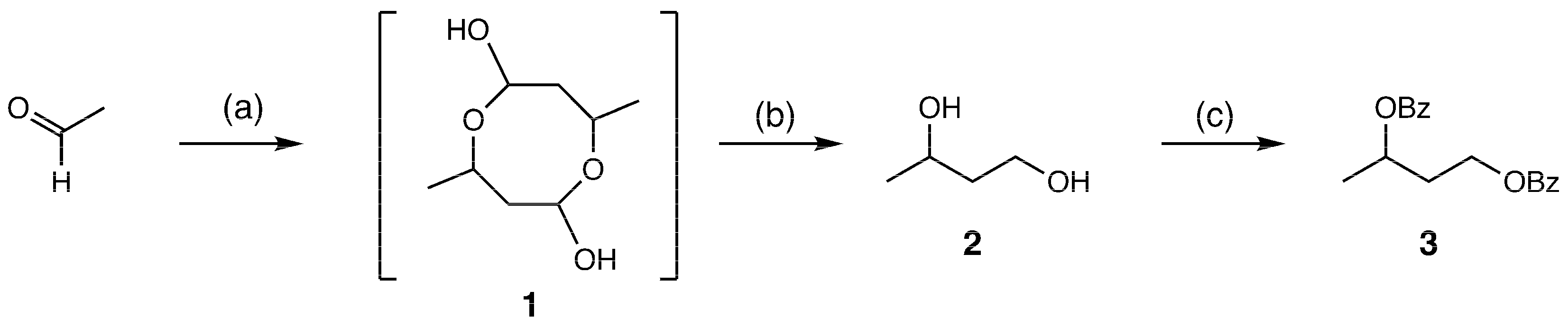
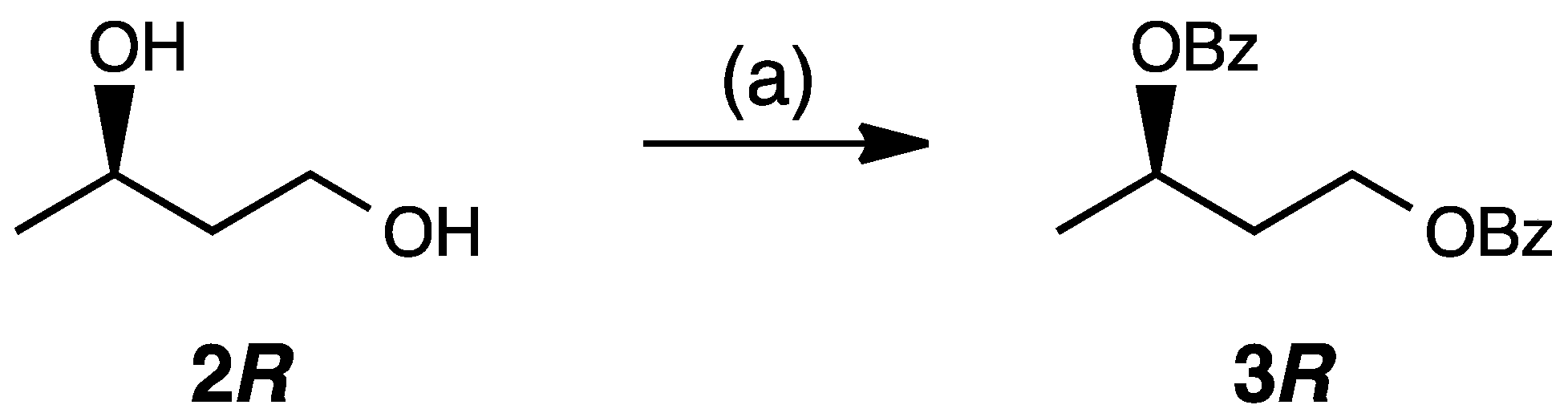
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis of 1,3-Butanediol Dibenzoate (3) from Acetaldehyde
3.3. Synthesis of (R)-1,3-Butanediol Dibenzoate (3R)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Cheali, P.; Quaglia, A.; Gernaey, K.V.; Sin, G. Effect of market price uncertainties on the design of optimal biorefinery systems—A systematic approach. Ind. Eng. Chem. Res. 2014, 53, 6021–6032. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Cutting-edge research for a greener sustainable future Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Long, X.; Ji, X. A review of the ecological and socioeconomic effects of biofuel and energy policy recommendations. Renew. Sustain. Energy Rev. 2016, 61, 41–52. [Google Scholar]
- Wang, K.; Ou, L.; Brown, T.; Brown, R.C. Beyond ethanol: A techno-economic analysis of an integrated corn biorefinery for the production of hydrocarbon fuels and chemicals. Biofuels Bioprod. Biorefin. 2014, 16, 177–189. [Google Scholar] [CrossRef]
- George, A.; Brandt, A.; Tran, K.; Zahavi, S.M.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Kataoka, N.; Vangnai, A.S.; Ueda, H.; Tajima, T.; Nakashimada, Y.; Kato, J. Enhancement of (R)-1, 3-butanediol production by engineered Escherichia coli using a bioreactor system with strict regulation of overall oxygen transfer coefficient and pH. Biosci. Biotechnol. Biochem. 2014, 78, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, A.; Yamamoto, H.; Kawada, N.; Kobayashi, Y. Industrial production of (R)-1,3-butanediol by new biocatalysts. J. Mol. Catal. B Enzym. 2001, 11, 513–521. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, G.; Wang, X.; Mu, X. Selective upgrading of ethanol with methanol in water for the production of improved biofuel—Isobutanol. Green Chem. 2016, 18, 2811–2818. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, Y.S.; Kim, D.W.; Rios, R.; Yang, J.W. Acetaldehyde: A small organic molecule with big impact on organocatalytic reactions. Chem. Eur. J. 2016, 22, 2214–2234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, A.; Rizvi, M.A.; Shah, B.A. Acetaldehyde in asymmetric organocatalytic transformations. RSC Adv. 2015, 5, 55926–55937. [Google Scholar] [CrossRef]
- Hayashi, Y.; Samanta, S.; Itoh, T.; Ishikawa, H. Asymmetric, catalytic, and direct self-aldol reaction of acetaldehyde catalyzed by diarylprolinol. Org. Lett. 2008, 10, 5581–5583. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Notz, W.; Barbas, C.F., III. Proline-catalyzed one-step asymmetric synthesis of 5-hydroxy-(2E)-hexenal from acetaldehyde. J. Org. Chem. 2002, 67, 301–303. [Google Scholar] [CrossRef] [PubMed]
| Entry | Reaction Condition | Isolated Yield of 1 |
|---|---|---|
| 1 | 100 mM Na phosphate buffer, pH 7 | not detected |
| 2 | 100 mM Na phosphate buffer, pH 8 | trace |
| 3 | 100 mM Na borate buffer, pH 9 | 25% |
| 4 | 100 mM Na borate buffer, pH 10 | 69% |
| 5 | 100 mM Na2HPO4–NaOH buffer, pH 11 | 31% |
| Entry | Concentration (M) | Reaction Time (h) | Isolated Yield of 1 |
|---|---|---|---|
| 1 | 0.5 | 48 | 35% |
| 2 | 1.0 | 48 | 43% |
| 3 | 2.0 | 48 | 49% |
| 4 | 3.0 | 48 | 61% |
| 5 | 4.0 | 48 | 67% |
| 6 | 5.0 | 24 | 54% |
| 7 | 5.0 | 48 | 69% |
| 8 | 5.0 | 72 | 62% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakamata, W.; Goto, K.; Ishiwata, S.; Ouki, R.; Sakai, R.; Iizuka, A.; Yano, S.; Hirano, T.; Nishio, T. 1,3-Butanediol Dibenzoate. Molbank 2016, 2016, M905. https://doi.org/10.3390/M905
Hakamata W, Goto K, Ishiwata S, Ouki R, Sakai R, Iizuka A, Yano S, Hirano T, Nishio T. 1,3-Butanediol Dibenzoate. Molbank. 2016; 2016(3):M905. https://doi.org/10.3390/M905
Chicago/Turabian StyleHakamata, Wataru, Kaho Goto, Sakiko Ishiwata, Rieka Ouki, Riku Sakai, Arisa Iizuka, Shuta Yano, Takako Hirano, and Toshiyuki Nishio. 2016. "1,3-Butanediol Dibenzoate" Molbank 2016, no. 3: M905. https://doi.org/10.3390/M905