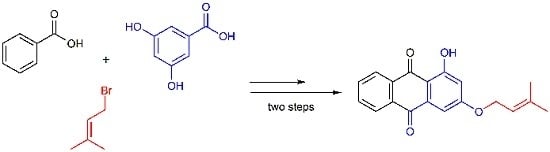
1-Hydroxy-3-(3-methylbut-2-enyloxy)anthracene-9,10-dione
Abstract
:1. Introduction
2. Experimental Section
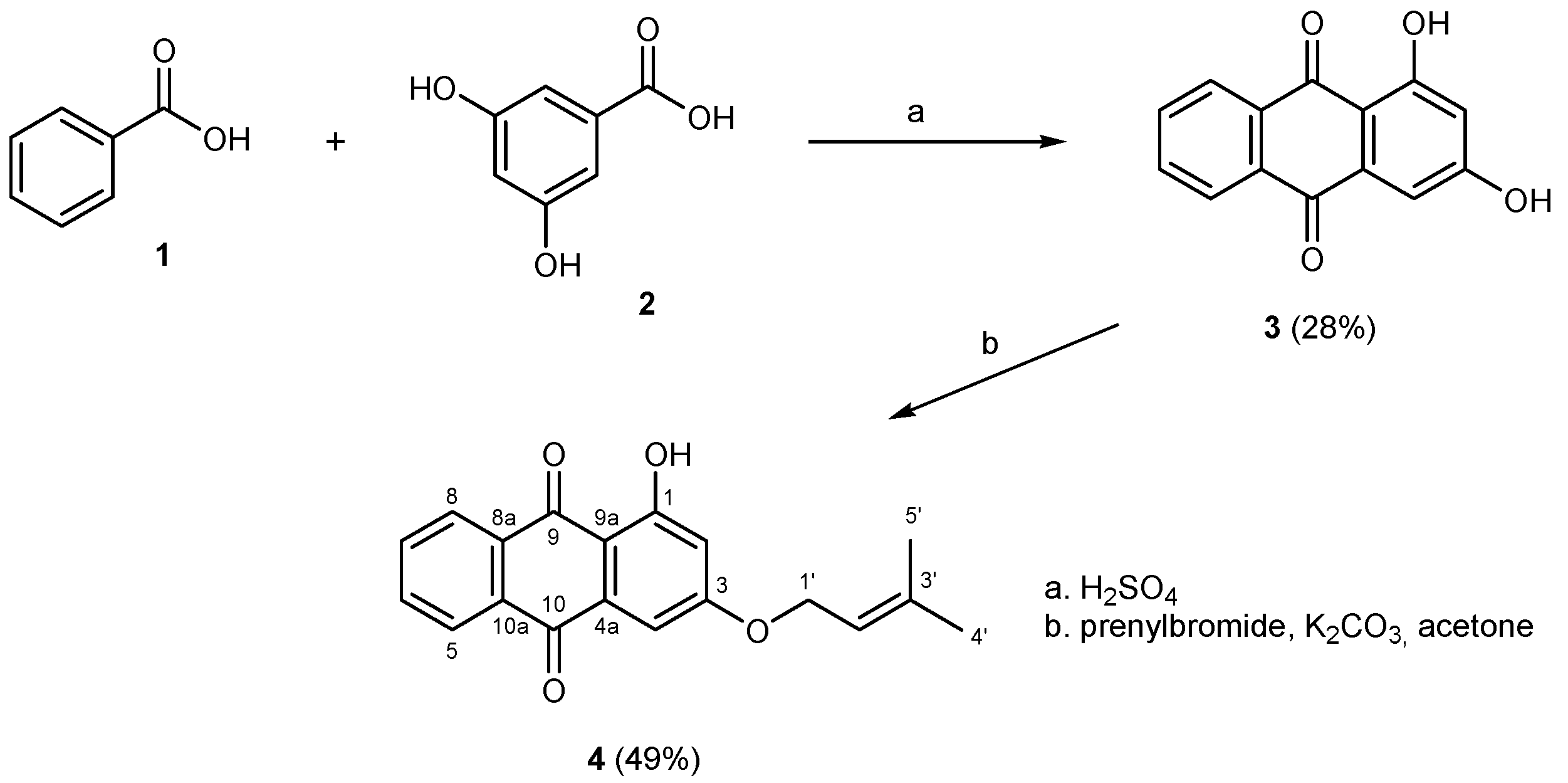
2.1. Synthesis of 1,3-Dihydroxyanthracene-9,10-dione (3)
2.2. Synthesis of 1-Hydroxy-3-(3-methylbut-2-enyloxy)anthracene-9,10-dione (4)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, R.; Geetanjali, G.; Chauhan, S.M.S. 9,10-Anthraquinones and other biologically active compounds from genus Rubia. Chem. Biodivers. 2004, 1, 1241–1264. [Google Scholar] [CrossRef] [PubMed]
- Epifano, F.; Genovese, S.; Menghini, L.; Curini, M. Chemistry and pharmacology of oxyprenylated secondary plantmetabolites. Phytochemistry 2007, 68, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.H.; Won, S.J.; Lin, C.N. Design, synthesis and cytotoxic effect of hydroxy- and 3-alkylaminopropoxy-9,10-anthraquinone erivatives. Bioorg. Med. Lett. 2005, 13, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Won, S.J.; Teng, C.H. 1,3-Dihydroxy-9,10-anthraquinone and 3-[(3-Amino)-propoxy]-9,10-anthraquinone Derivatives and Pharmaceutical Compositions Comprising the Same. U.S. Patent 20080027141 A1, 31 January 2008. [Google Scholar]
- Murschell, A.E.; Sutherland, T.C. Anthraquinone-based discotic liquid crystals. Langmuir 2010, 26, 12859–12866. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurbayti, S.; Mujahidin, D.; Syah, Y.M. 1-Hydroxy-3-(3-methylbut-2-enyloxy)anthracene-9,10-dione. Molbank 2016, 2016, M888. https://doi.org/10.3390/M888
Nurbayti S, Mujahidin D, Syah YM. 1-Hydroxy-3-(3-methylbut-2-enyloxy)anthracene-9,10-dione. Molbank. 2016; 2016(1):M888. https://doi.org/10.3390/M888
Chicago/Turabian StyleNurbayti, Siti, Didin Mujahidin, and Yana M. Syah. 2016. "1-Hydroxy-3-(3-methylbut-2-enyloxy)anthracene-9,10-dione" Molbank 2016, no. 1: M888. https://doi.org/10.3390/M888