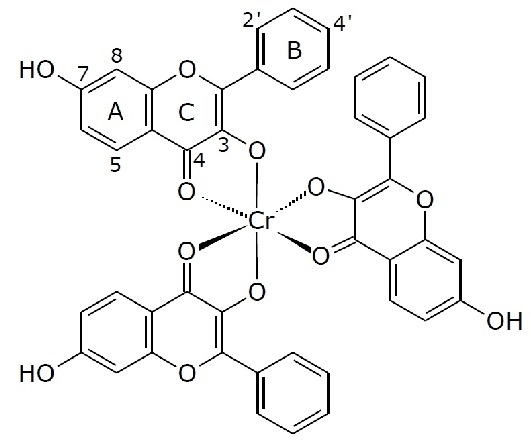
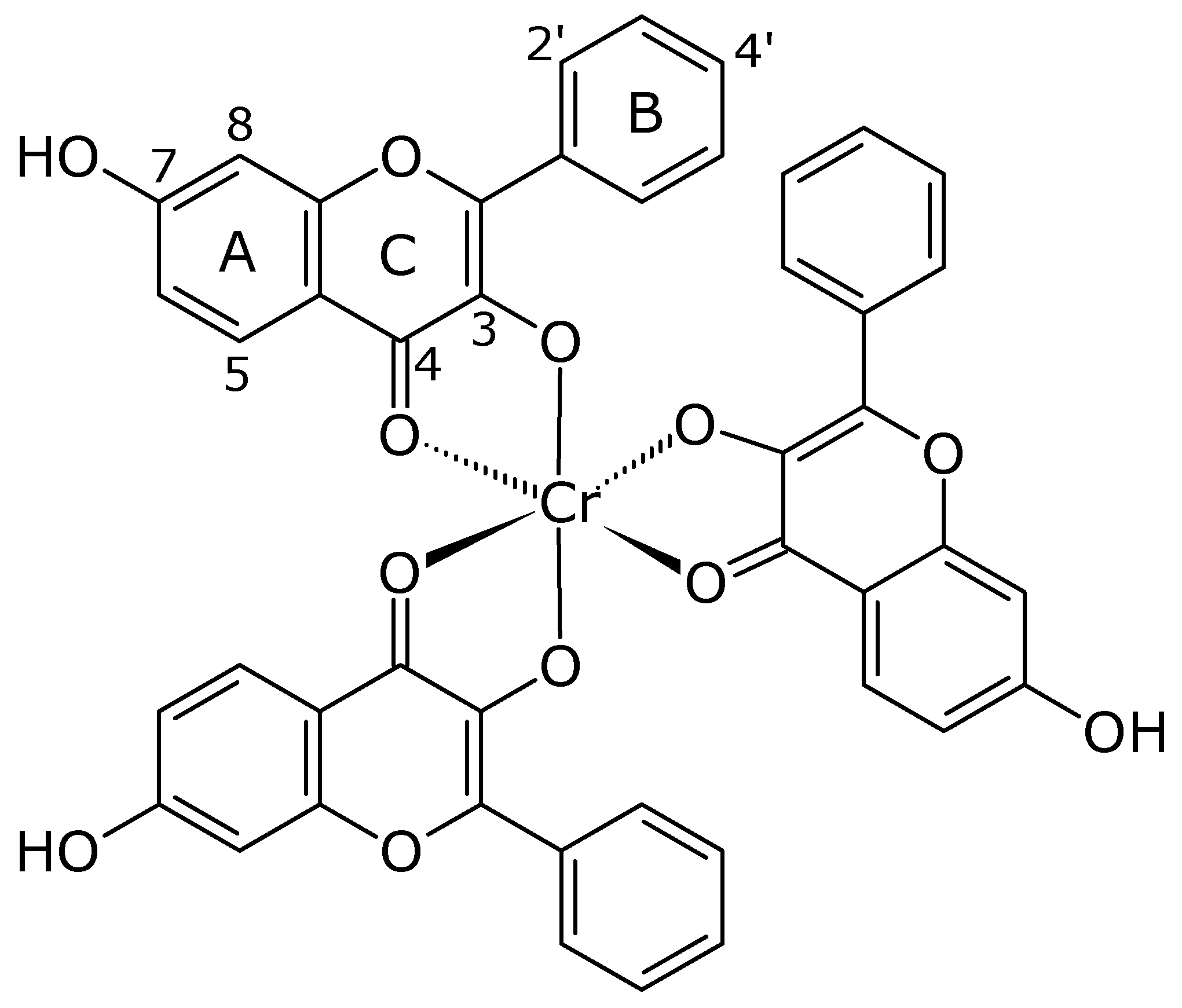
Tris(3,7-dihydroxyflavonolate-κO3,O4)chromium(III) Complex
Abstract
:1. Introduction
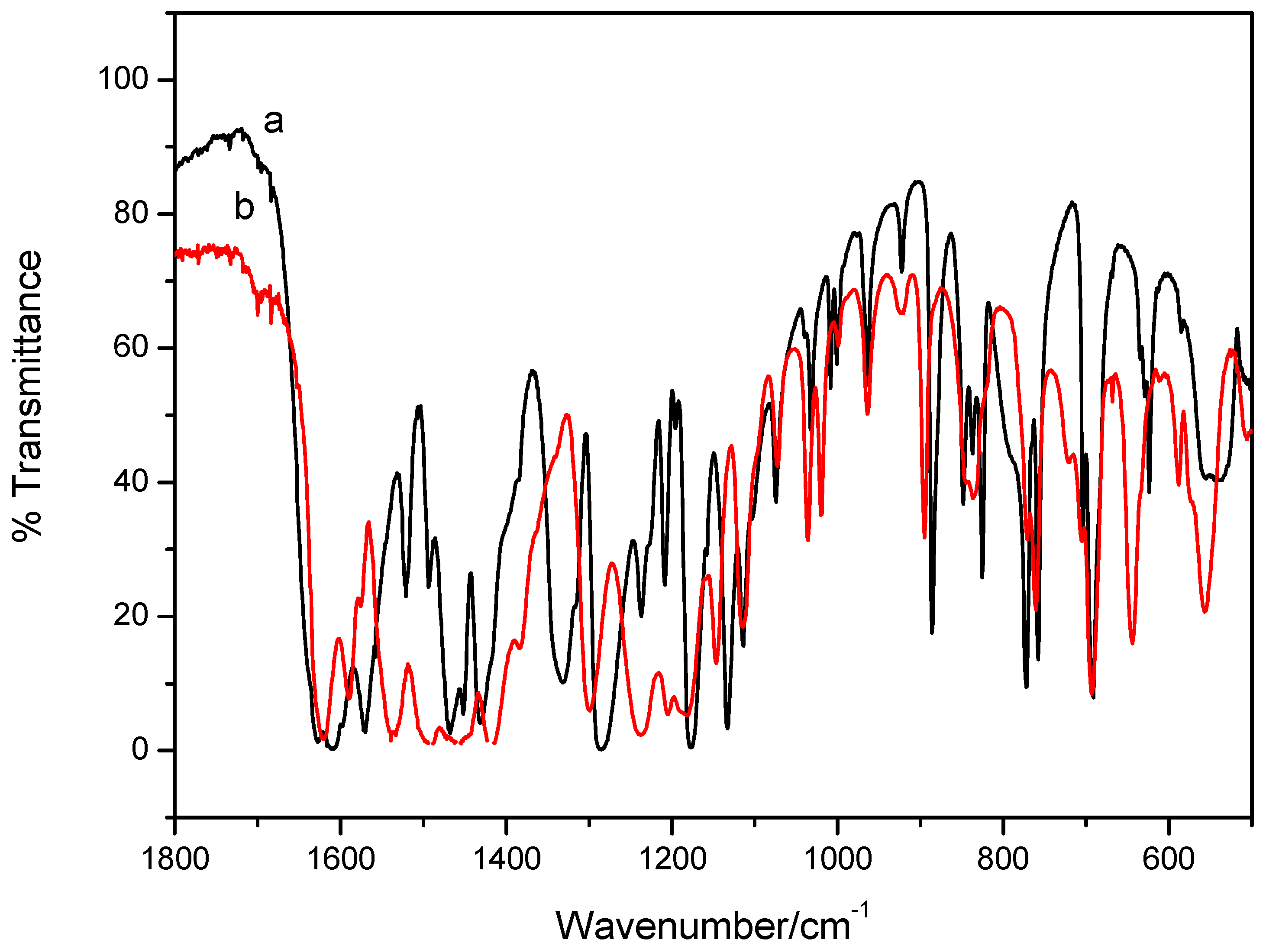
2. Results and Discussion

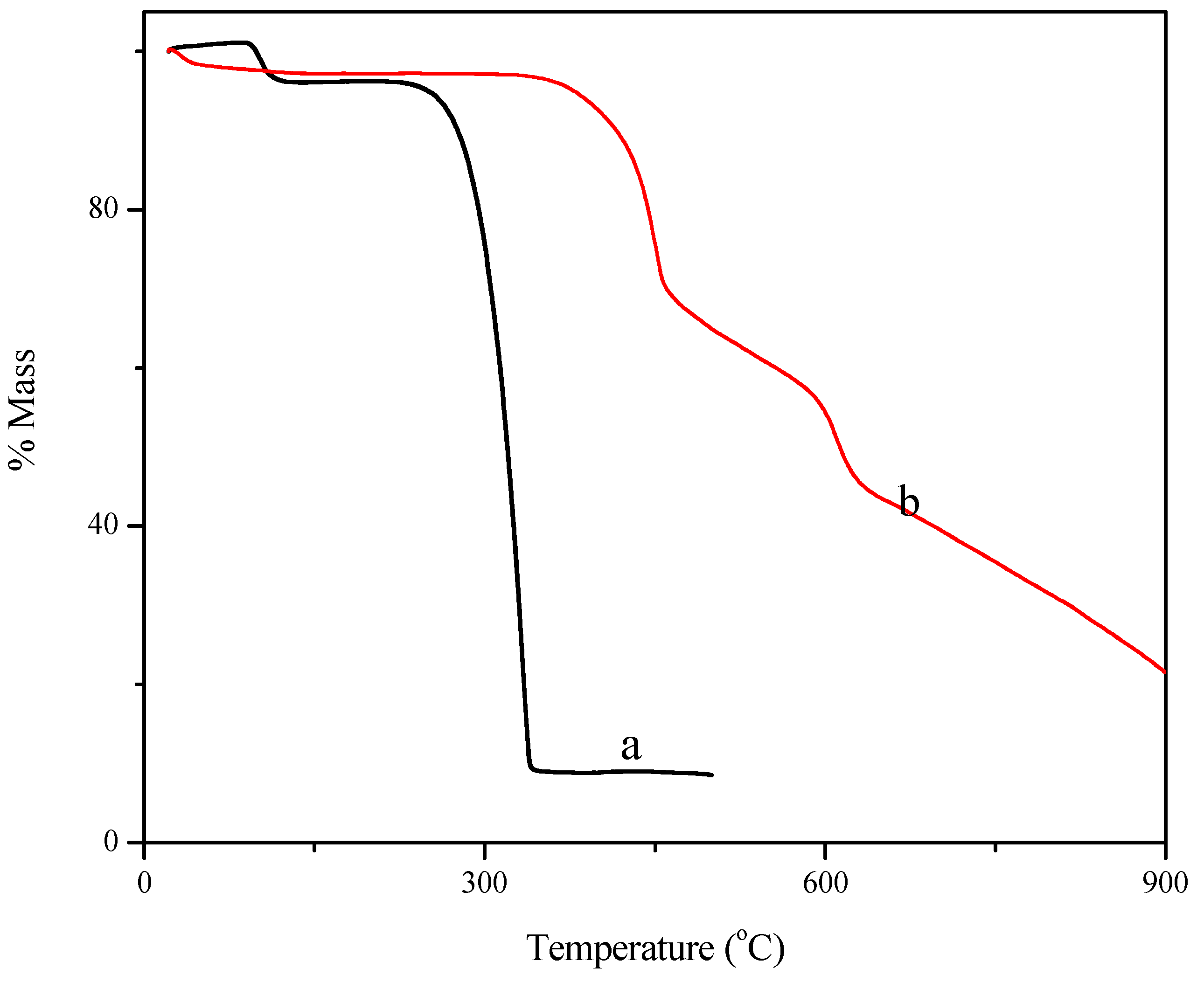

3. Experimental Section
Tris(3,7-dihydroxyflavonolate-κO3,O4)chromium(III) Complex Synthesis
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [PubMed]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid-metal ion complexes: A novel class of therapeutic agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, A.D.P.; Decandio, C.C.; Almeida, S.N.; Ferreira, M.J.P.; Romoff, P.; Rocha, R.C. A trinuclear oxo-chromium(III) complex containing the natural flavonoid primuletin: Synthesis, characterization, and antiradical properties. Molecules 2015, 20, 6310–6318. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, S.; Cao, W.; Liang, Y.; Song, J. Antioxidant property of quercetin–Cr(III) complex: The role of Cr(III) ion. J. Mol. Struct. 2009, 918, 194–197. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Li, T. Synthesis, characterization, antioxidative activity and DNA binding properties of the Copper(II), Zinc(II), Nickel(II) complexes with 1,2-Di(4′-iminonaringenin)ethane. Chem. Pharm. Bull. 2008, 56, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.S.; Andrades, N.E.D.; Paulino, N.; Sawaya, A.C.H.F.; Eberlin, M.N.; Marcucci, M.C.; Favero, G.M.; Novak, E.M.; Bydlowski, S.P. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.V.; Giovani, W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Afanas’eva, I.B.; Ostrakhovitch, E.A.; Mikhal’chik, E.V.; Ibragimova, G.A.; Korkina, L.G. Enhancement of antioxidant and anti-inflammatory activities of bioflavonoid rutin by complexation with transition metals. Biochem. Pharmacol. 2001, 61, 677–684. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Chen, L.; Xie, Y.; Wang, L.Y.; Xie, M.X. Spectroscopic investigation on the interaction of 3,7-dihydroxyflavone with different isomers of human serum albumin. Food Chem. 2012, 132, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Iron complex nanoparticles synthesized by Eucalyptus leaves. ACS Sustain. Chem. Eng. 2013, 1, 1551–1554. [Google Scholar] [CrossRef]
- Gupta, S.K.; Anjana, C.; Sen, N.; Butcher, R.J.; Jasinski, J.P. Synthesis, crystal structure, DFT and spectroscopic studies of mononuclear chromium(III) complex with bidentate ligand. Polyhedron 2012, 43, 8–14. [Google Scholar] [CrossRef]
- Wang, M.; Teslova, T.; Xu, F.; Spataru, T.; Lombardi, J.R.; Birke, R.L.; Leona, M. Raman and surface enhanced raman scattering of 3-hydroxyflavone. J. Phys. Chem. C 2007, 111, 3038–3043. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexiou, A.D.P.; Silva, D.D.E.; Romoff, P.; Ferreira, M.J.P. Tris(3,7-dihydroxyflavonolate-κO3,O4)chromium(III) Complex. Molbank 2016, 2016, M886. https://doi.org/10.3390/M886
Alexiou ADP, Silva DDE, Romoff P, Ferreira MJP. Tris(3,7-dihydroxyflavonolate-κO3,O4)chromium(III) Complex. Molbank. 2016; 2016(1):M886. https://doi.org/10.3390/M886
Chicago/Turabian StyleAlexiou, Anamaria D. P., Denny D. E. Silva, Paulete Romoff, and Marcelo J. P. Ferreira. 2016. "Tris(3,7-dihydroxyflavonolate-κO3,O4)chromium(III) Complex" Molbank 2016, no. 1: M886. https://doi.org/10.3390/M886