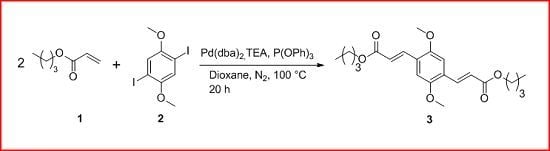
1,4-Di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General Information
3.2. 1,4-Di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, J.M.; Yang, S.; Shim, S.E.; Choe, S. Synthesis of poly(acrylamide-co-divinylbenzene) microspheres by precipitation polymerization. J. Polym. Sci. Part A: Polym. Chem. 2005, 43, 5343–5346. [Google Scholar] [CrossRef]
- Donescu, D.; Raditoiu, V.; Spataru, C.I.; Somoghi, S.; Ghiurea, M.; Radovici, C.; Fierascu, R.C.; Schinteie, G.; Leca, A.; Kuncser, V. Superparamagnetic magnetite–divinylbenzene–maleic anhydride copolymer nanocomposites obtained by dispersion polymerization. Eur. Polym. J. 2012, 48, 1709–1716. [Google Scholar] [CrossRef]
- Weber, V.; Linsberger, I.; Hauner, M.; Leistner, A.; Leistner, A.; Falkenhagen, D. Neutral Styrene Divinylbenzene Copolymers for Adsorption of Toxins in Liver Failure. Biomacromolecules 2008, 9, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yu, Y.; Nguyen, S.T.; Hupp, J.T.; Broadbelt, L.J.; Farha, O.K. Epoxidation of the commercially relevant divinylbenzene with [tetrakis-(pentafluorophenyl)porphyrinato]iron(III) chloride and its derivatives. Ind. Eng. Chem. Res. 2015, 54, 922–927. [Google Scholar] [CrossRef]
- Durie, S.; Jerabek, K.; Mason, C.; Sherrington, D.C. One-pot synthesis of branched poly(styrene-divinylbenzene) suspension polymerized resins. Macromolecules 2002, 35, 9665–9672. [Google Scholar] [CrossRef]
- Chen, B.; Wang, W.; Ma, X.; Wang, C.; Li, R. Adsorption behaviors of glycerol from biodiesel on sulfonated polystyrene−divinylbenzene resins in different forms. Energ. Fuels 2012, 26, 7060–7067. [Google Scholar] [CrossRef]
- Bertran, O.; Curcó, D.; Torras, J.; Ferreira, A.; Alemán, C. Field-induced transport in sulfonated poly(styrene-co-divinylbenzene) membranes. Macromolecules 2010, 43, 10521–10527. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, H. Synthesis, electrochemistry, and electroluminescence of novel red-emitting poly(p-phenylenevinylene) derivative with 2-pyran-4-ylidene-malononitrile obtained by the Heck reaction. Chem. Mater. 2002, 14, 2270–2275. [Google Scholar] [CrossRef]
- Chen, J.T.; Hsu, C.S. Poly(2,3-diphenyl-1,4-phenylenevinylene) (DP-PPV) derivatives: Synthesis, properties, and their applications in polymer light-emitting diodes. Polymer 2013, 54, 4045–4058. [Google Scholar] [CrossRef]
- Gao, W.; Yan, M.; Ge, S.; Liu, X.; Yu, J. Fluorescent sensor based on a novel conjugated polyfluorene derivative. Spectrochim. Acta Part A 2012, 95, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.T.; Son, H.S.; Ku, J.K.; Kim, B.H. Synthesis and photophysical studies of bis-enediynes as tunable fluorophores. J. Am. Chem. Soc. 2003, 125, 11241–11248. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.T.; Son, H.S.; Ku, J.K.; Kim, B.H. Novel fluorophores: Efficient synthesis and photophysical study. Org. Lett. 2001, 3, 2469–2471. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.T.; Kim, B.H. π-Conjugated dendrimers based on bis(enediynyl)benzene units. Org. Lett. 2004, 6, 2669–2672. [Google Scholar] [CrossRef] [PubMed]
- Kaafarani, B.R.; Wex, B.; Wang, F.; Catanescu, O.; Chien, L.C.; Neckers, D.C. Synthesis of highly fluorescent Y-enyne dendrimers with four and six arms. J. Org. Chem. 2003, 68, 5377–5380. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M. One hundred years of helicene chemistry. Part 1: Non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 2013, 42, 968–1006. [Google Scholar] [CrossRef] [PubMed]
- List, B.; Doehring, A.; Fonseca, m.T.H.; Wobser, K.; Van-Thienen, H.; Torres, R.R.; Galilea, P.L. Practical synthesis of (E)-α,β-unsaturated esters from aldehydes. Adv. Synth. Catal. 2005, 347, 1558–1560. [Google Scholar] [CrossRef]
- Kilway, K.V.; Siegel, J.S. Control of functional group proximity and direction by conformational networks: Synthesis and stereodynamics of persubstituted arenes. Tetrahedron 2001, 57, 3615–3627. [Google Scholar] [CrossRef]
- Irngartinger, H.; Herpich, R. Synthesis and topochemistry of 2,5-bisacrylate-substituted 1,4-benzoquinones. Eur. J. Org. Chem. 1998, 595–604. [Google Scholar] [CrossRef]
- Vatèle, J.-M. One-pot selective oxidation/olefination of primary alcohols using TEMPO–BAIB system and stabilized phosphorus ylides. Tetrahedron Lett. 2006, 47, 715–718. [Google Scholar] [CrossRef]
- Wang, A.-E.; Xie, J.-H.; Wang, L.-X.; Zhou, Q.-L. Triaryl phosphine-functionalized N-heterocyclic carbene ligands for Heck reaction. Tetrahedron 2005, 61, 259–266. [Google Scholar] [CrossRef]
- Cui, X.; Li, Z.; Tao, C.-Z.; Xu, Y.; Li, J.; Liu, L.; Guo, Q.-X. N,N-Dimethyl-β-alanine as an inexpensive and efficient ligand for palladium-catalyzed Heck reaction. Org. Lett. 2006, 8, 2467–2470. [Google Scholar] [PubMed]
- Dai, M.; Liang, B.; Wang, C.; Chen, J.; Yang, Z. Synthesis of a novel C2-symmetric thiourea and its application in the Pd-catalyzed cross-coupling reactions with arenediazonium salts under aerobic conditions. Org. Lett. 2004, 6, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kanbara, M.; Ohashi, M.; Hayase, S.; Kawatsura, M.; Kato, T.; Miyazawa, K.; Takagi, Y.; Uno, H. gem-Difluorocyclopropane as core molecule candidate for liquid crystal compounds. J. Fluorine Chem. 2007, 128, 1112–1120. [Google Scholar] [CrossRef]
- Sun, W.; Yu, B.; Kühn, F.E. Ruthenium(II)–salen complexes-catalyzed olefination of aldehydes with ethyl diazoacetate. Tetrahedron Lett. 2006, 47, 1993–1996. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Korotchenko, V.N.; Shastin, A.V.; Balenkova, E.S. Catalytic olefination of carbonyl compounds. A new versatile method for the synthesis of alkenes. Russ. Chem. Bull. 2004, 53, 1034–1064. [Google Scholar] [CrossRef]
- Oh, C.H.; Lim, Y.M.; You, C.H. Platinum-catalyzed cross-couplings of organoboronic acids with aryl iodides. Tetrahedron Lett. 2002, 43, 4645–4647. [Google Scholar] [CrossRef]
- Katayama, H.; Nagao, M.; Nishimura, T.; Matsui, K.; Umeda, K.; Akamatsu, K.; Tsuruoka, T.; Nawafune, H.; Ozawa, F. Stereocontrolled synthesis and optical properties of all-cis poly(phenylene vinylenes) (PPVs): A method for direct patterning of PPVs. J. Am. Chem. Soc. 2005, 127, 4350–4353. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.A.; Lahti, P.M. A photoluminescent, segmented oligo-polyphenylenevinylene copolymer with hydrogen-bonding pendant chains. Chem. Mater. 2004, 16, 55–61. [Google Scholar] [CrossRef]

© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molano, W.A.; Sierra, C.; Ochoa-Puentes, C. 1,4-Di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene. Molbank 2015, 2015, M876. https://doi.org/10.3390/M876
Molano WA, Sierra C, Ochoa-Puentes C. 1,4-Di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene. Molbank. 2015; 2015(4):M876. https://doi.org/10.3390/M876
Chicago/Turabian StyleMolano, William A., Cesar Sierra, and Cristian Ochoa-Puentes. 2015. "1,4-Di(2-butoxycarbonyl-trans-vinyl)-2,5-dimethoxybenzene" Molbank 2015, no. 4: M876. https://doi.org/10.3390/M876