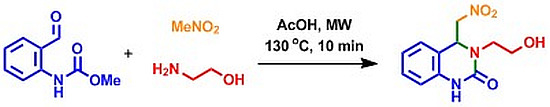
3,4-Dihydro-3-(2-hydroxyethyl)-4-(nitromethyl)quinazolin-2(1H)-one
Abstract
:Experimental Section
General
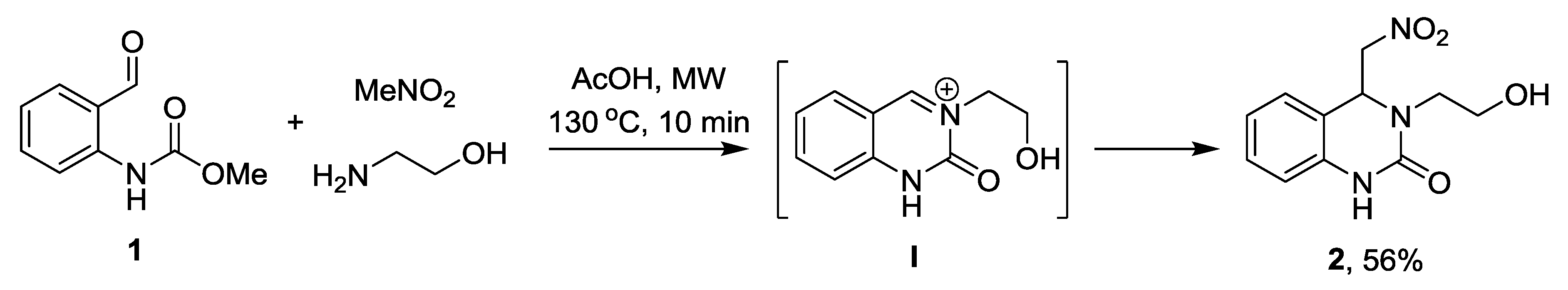
Experimental Procedure for the Preparation of 3,4-Dihydro-3-(2-hydroxyethyl)-4-(nitromethyl)quinazolin-2(1H)-one (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Corbett, J.W.; Ko, S.S.; Rodgers, J.D.; Gearhart, L.A.; Magnus, N.A.; Bacheler, L.T.; Diamond, S.; Jeffrey, S.; Klabe, R.M.; Cordova, B.C.; et al. Inhibition of Clinically Relevant Mutant Variants of HIV-1 by Quinazolinone Non-Nucleoside Reverse Transcriptase Inhibitors. J. Med. Chem. 2000, 43, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Mishra, A.K.; Bajpai, A.; Mishra, P.; Sharma, R.K.; Pandey, V.K.; Singh, V.K. Synthesis and Pharmacological Study of Novel Pyrido-quinazolone Analogues as Anti-fungal, Antibacterial, and Anticancer Agents. Bioorg. Med. Chem. Lett. 2006, 16, 4581–4585. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Muraoka, M.; Matsui, K.; Kojima, A. Discovery of a Novel Potent Na+/Ca2+ Exchanger Inhibitor: Design, Synthesis and Structure-Activity Relationships of 3,4-Dihydro-2(1H)-Quinazolinone Derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 3471–3475. [Google Scholar] [CrossRef]
- Barrow, J.C.; Rittle, K.E.; Reger, T.S.; Yang, Z.-Q.; Bondiskey, P.; McGaughey, G.B.; Bock, M.G.; Hartman, G.D.; Tang, C.; Ballard, J.; et al. Discovery of 4,4-Disubstituted Quinazolin-2-ones as T-Type Calcium Channel Antagonists. ACS Med. Chem. Lett. 2010, 1, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kosasayama, A.; Higashi, K.I.F. Cyclic Guanidines VI. Synthesis of Hypoglycemic Tricyclic Guanidines. Chem. Pharm. Bull. 1979, 27, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.Y.H. Synthetic Studies on Quinazoline Derivatives II. The Reactions of 2-Trichloro-and 2-Trifluoroacetamidobenzophenones with Primary Amines. Chem. Pharm. Bull. 1981, 29, 2135–2156. [Google Scholar] [CrossRef]
- Noble, A.; Anderson, J.C. Nitro-Mannich Reaction. Chem. Rev. 2013, 113, 2887–2939. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, Y.; Zhang, S.; Chen, X.; Wang, W. Bifunctional Cinchona Alkaloid Thiourea Catalyzed Highly Efficient, Enantioselective Aza-Henry Reaction of Cyclic Trifluoromethyl Ketimines: Synthesis of Anti-HIV Drug DPC 083. Angew. Chem. Int. Ed. 2011, 50, 11773–11776. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.Y.; Wieckowski, K.; Wu, P.; Sawant, R.T.; Odell, L.R. A Microwave-Assisted Multicomponent Synthesis of Substituted 3,4-Dihydroquinazolinones. Org. Biomol. Chem. 2015, 13, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawant, R.T.; Stevens, M.Y.; Odell, L.R. 3,4-Dihydro-3-(2-hydroxyethyl)-4-(nitromethyl)quinazolin-2(1H)-one. Molbank 2015, 2015, M866. https://doi.org/10.3390/M866
Sawant RT, Stevens MY, Odell LR. 3,4-Dihydro-3-(2-hydroxyethyl)-4-(nitromethyl)quinazolin-2(1H)-one. Molbank. 2015; 2015(3):M866. https://doi.org/10.3390/M866
Chicago/Turabian StyleSawant, Rajiv T., Marc Y. Stevens, and Luke R. Odell. 2015. "3,4-Dihydro-3-(2-hydroxyethyl)-4-(nitromethyl)quinazolin-2(1H)-one" Molbank 2015, no. 3: M866. https://doi.org/10.3390/M866