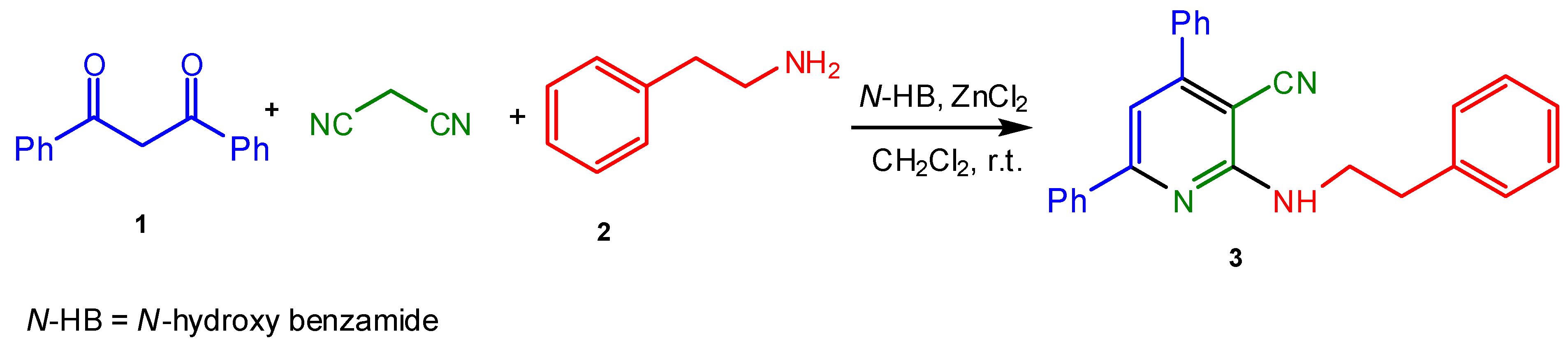
3-Cyano-4,6-diphenyl-2-(phenethylamino)pyridine
Abstract
:Experimental
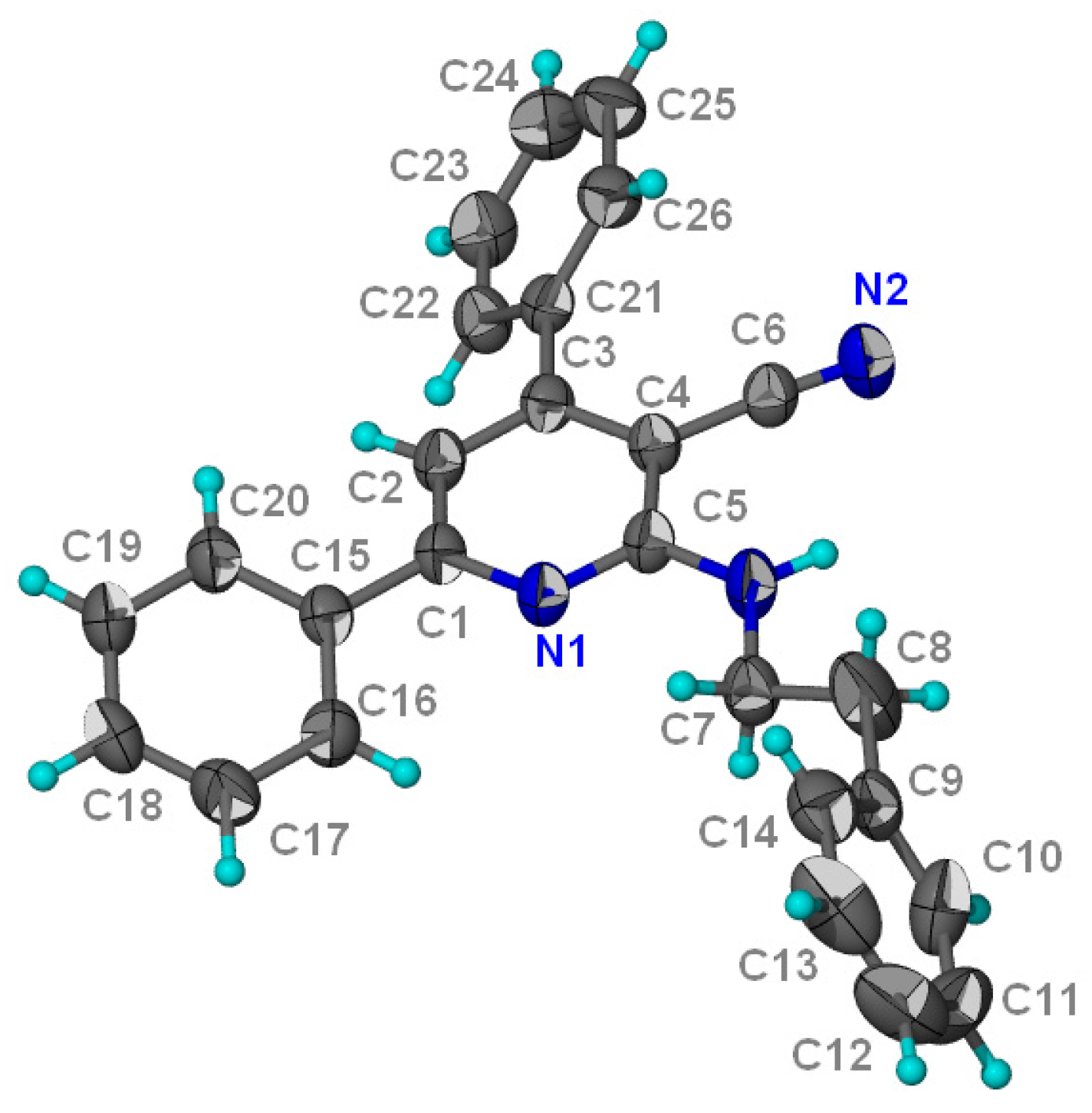
Structural Characterization
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Duarte, F. Solid-state multiple-prism grating dye-laser oscillator. J. Appl. Opt. 1994, 33, 3857–3860. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of fluorescence spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Stoltefuss, J.; Goldmann, S.; Straub, A.; Boshagen, H.; Bechem, M.; Gross, R.; Hebisch, S.; Hutter, J.; Rounding, H.P. Process for Preparing 1,4-Dihydro-2-amino-3-carboxy-5-cyano-pyridine Derivatives. U.S. Patent 5432282 A, 11 July 1995. [Google Scholar]
- Henry, G.D. De novo synthesis of substituted pyridines. Tetrahedron 2004, 60, 6043–6061. [Google Scholar] [CrossRef]
- Queiroz, M.R.P.; Ferreira, I.C.F.R.; Calhelha, R.C.; Estevinho, L.M. Synthesis and antioxidant activity evaluation of new 7-aryl or 7-heteroarylamino-2,3-dimethyl benzo[b]thiophenes obtained by Buchwald–Hartwig C–N cross-coupling. Bioorg. Med. Chem. 2007, 15, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Manna, F.; Chimenti, F.; Bolasco, A.; Bizzarri, B.; Filippelli, W.; Filippelli, A.; Gagliardi, L. Anti-inflammatory, analgesic and antipyretic 4,6-disubstituted 3-cyano-2-aminopyridines. Eur. J. Med. Chem. 1999, 34, 245–254. [Google Scholar] [CrossRef]
- Deng, J.; Sanchez, T.; Al-Mawsawi, L.Q.; Dayam, R.; Yunes, R.A.; Garofalo, A.; Bolger, M.B.; Neamati, N. Discovery of structurally diverse HIV-1 integrase inhibitors based on a chalcone pharmacophore. Bioorg. Med. Chem. 2007, 15, 4985–5002. [Google Scholar] [CrossRef] [PubMed]
- Bomika, Z.; Andaburskaya, M.; Pelcher, Y.É.; Dubur, G.Y. Some nucleophilic substitution reactions of 2-chloro-3-cyanopyridines. Chem. Heterocycl. Compd. 1976, 12, 896–899. [Google Scholar] [CrossRef]
- Kambe, S.; Saito, K.; Sakurai, A.; Midorikawa, H. A simple method for the preparation of 2-amino-4-aryl-3-cyanopyridines by the condensation of malononitrile with aromatic aldehydes and alkyl ketones in the presence of ammonium acetate. Synthesis 1980, 1980, 366–368. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Yao, Y.; Zhang, L.; Wang, W. One-pot synthesis of 2-amino-3-cyanopyridine derivatives catalyzed by ytterbium perfluorooctanoate [Yb(PFO)3]. Tetrahedron Lett. 2011, 52, 509–511. [Google Scholar] [CrossRef]
- Xin, X.; Huang, P.; Xiang, D.; Zhang, R.; Zhao, F.; Zhang, N.; Dong, D. [5C+ 1N] Annulation of 2,4-pentadienenitriles with hydroxylamine: A synthetic route to multi-substituted 2-aminopyridines. Org. Biomol. Chem. 2013, 11, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Zonouzi, A.; Izakian, Z.; Ng, S.W. Novel synthesis of some new fluorescent 2-amino-3-cyanopyridines. Heterocycles 2012, 85, 2713–2721. [Google Scholar] [CrossRef]
- Zonouzi, A.; Mirzazadeh, R.; Peivandi, A.; Dehdari, S. Efficient synthesis of N-substituted 4-arylquinoline derivatives using ZnCl2 or ZrO2. Heterocycles 2012, 85, 1447–1456. [Google Scholar] [CrossRef]
- Zonouzi, A.; Mirzazadeh, R.; Ng, S.W. Trimethyl 4,6-Dicyano-5-hydroxybenzene-1,2,3-tricarboxylate. Molbank 2012, 2012, M775. [Google Scholar] [CrossRef]
- Crystallographic data for the structure of compound 3 reported in this paper have been deposited with the Cambridge Crystallographie Data Center as supplementary publication No. 983903. CCDC 983903 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.com.ac.uk/data_request/cif. (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: [email protected]).
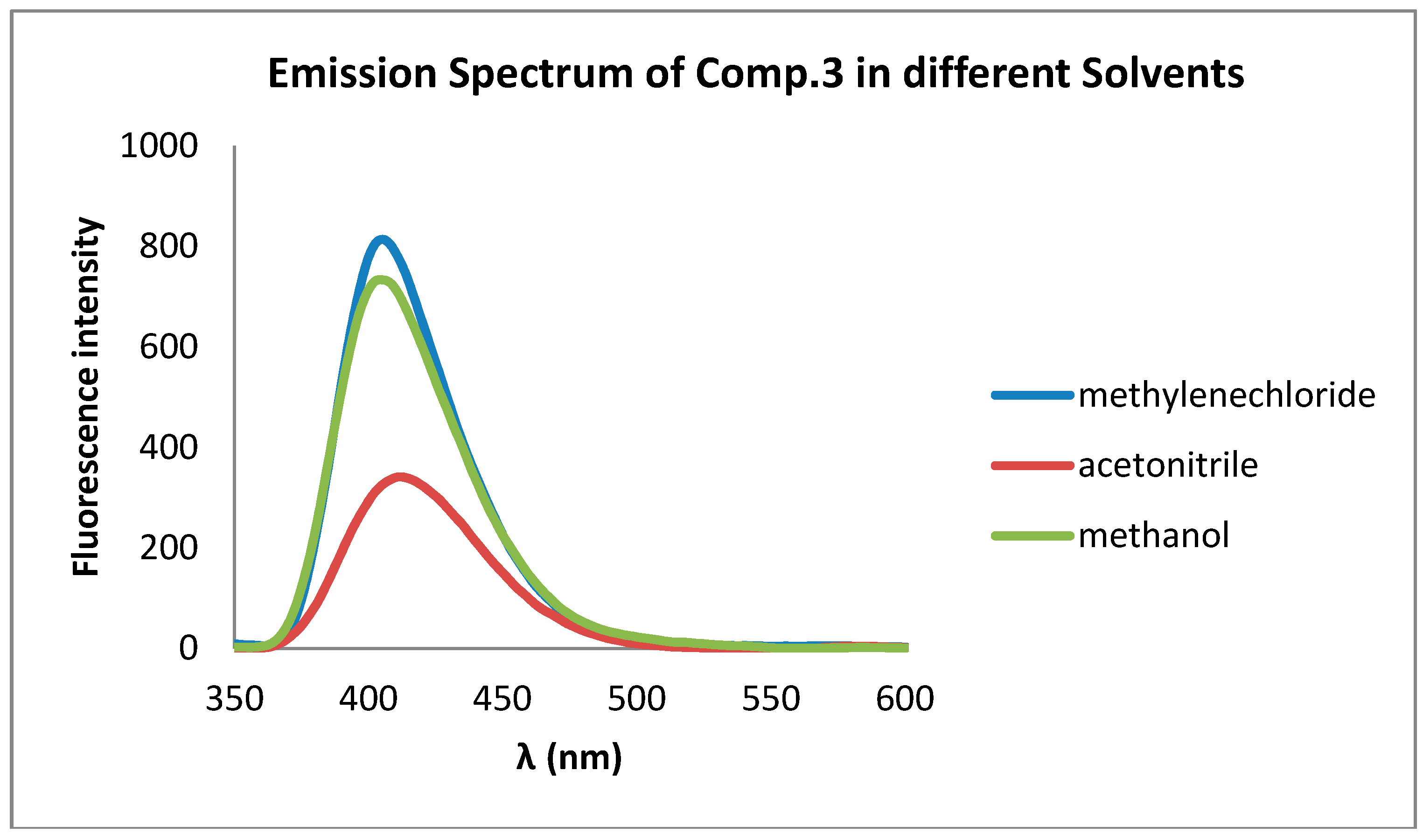
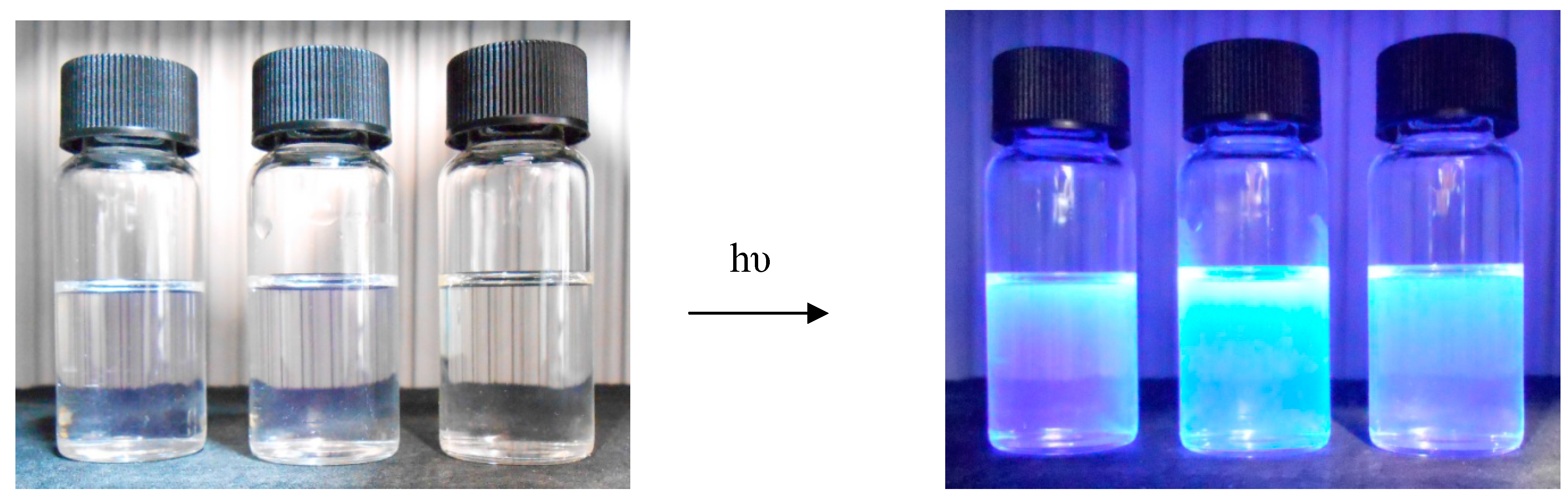

| CH3OH | CH3CN | CH2Cl2 | ||||
| Comp | λAbs | λFlu | λAbs | λFlu | λAbs | λFlu |
| 3 | 300 | 404 | 300 | 403 | 320 | 401 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zonouzi, A.; Izakian, Z.; Salehi, H.; Abdi, K.; Ng, S.W. 3-Cyano-4,6-diphenyl-2-(phenethylamino)pyridine. Molbank 2014, 2014, M831. https://doi.org/10.3390/M831
Zonouzi A, Izakian Z, Salehi H, Abdi K, Ng SW. 3-Cyano-4,6-diphenyl-2-(phenethylamino)pyridine. Molbank. 2014; 2014(3):M831. https://doi.org/10.3390/M831
Chicago/Turabian StyleZonouzi, Afsaneh, Zakieh Izakian, Hasan Salehi, Khosrou Abdi, and Seik Weng Ng. 2014. "3-Cyano-4,6-diphenyl-2-(phenethylamino)pyridine" Molbank 2014, no. 3: M831. https://doi.org/10.3390/M831





