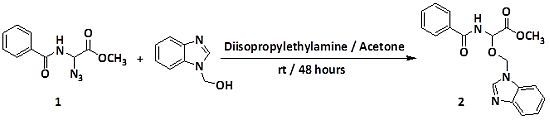
Methyl 2-Benzamido-2-(1H-benzimidazol-1-ylmethoxy)acetate
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Ebenso, E.E.; Isabirye, D.A.; Eddy, N.O. Adsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic medium. Int. J. Mol. Sci. 2010, 11, 2473–2498. [Google Scholar] [CrossRef] [PubMed]
- Eddy, N.O.; Ebenso, E.E.; Ibok, U.J. Adsorption, synergistic inhibitive effect and quantum chemical studies of ampicillin (AMP) and halides for the corrosion of mild steel in H2SO4. J. Appl. Electrochem. 2010, 40, 445–456. [Google Scholar] [CrossRef]
- Eddy, N.O.; Ibok, U.J.; Ebenso, E.E.; El Nemr, A.; El Ashry, E.S.H. Quantum chemical study of the inhibition of the corrosion of mild steel in H2SO4 by some antibiotics. J. Mol. Model. 2009, 15, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Eddy, N.O. Experimental and theoretical studies on some amino acids and their potential activity as inhibitors for the corrosion of mild steel, part 2. J. Adv. Res. 2011, 2, 35–47. [Google Scholar] [CrossRef]
- Li, J.; Shi, R.; Yang, C.; Zhu, X. Exploration of the binding of benzimidazole-biphenyl derivatives to hemoglobin using docking and molecular dynamics simulation. Int. J. Biol. Macromol. 2011, 48, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Goebel, M.; Staels, B.; Unger, T.; Kintscher, U.; Gust, R. Characterization of new PPARgamma agonists: Benzimidazole derivatives - the importance of position 2. Chem. Med. Chem. 2009, 4, 1142–1136. [Google Scholar] [CrossRef] [PubMed]
- Moss, N.; Choi, Y.; Cogan, D.; Flegg, A.; Kahrs, A.; Loke, P.; Meyn, O.; Nagaraja, R.; Napier, S.; Parker, A.; et al. A new class of 5-HT2B antagonists possesses favorable potency, selectivity, and rat pharmacokinetic properties. Bioorg. Med. Chem. Lett. 2009, 19, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, S.; Kayagil, I.; Yurttas, L. Microwave supported synthesis of some novel 1,3-Diarylpyrazino[1,2-a]benzimidazole derivatives and investigation of their anticancer activities. Eur. J. Med. Chem. 2011, 46, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Law, P.K.; de Gasparo, M.; Leung, P.S. Combination of the Dipeptidyl Peptidase IV Inhibitor LAF237 [(S)-1-[(3-Hydroxy-1-adamantyl)ammo]acetyl-2-cyanopyrrolidine] with the Angiotensin II Type 1 Receptor Antagonist Valsartan [N-(1-Oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]methyl]-L-valine] Enhances Pancreatic Islet Morphology and Function in a Mouse Model of Type 2 Diabetes. J. Pharmacol. Exp. Ther. 2008, 327, 683–691. [Google Scholar] [PubMed]
- Pfeffer, M.A.; McMurray, J.J.; Velazquez, E.J.; Rouleau, J.L.; Køber, L.; Maggioni, A.P.; Solomon, S.D.; Swedberg, K.; van de Werf, F.; White, H.; et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N. Engl. J. Med. 2003, 349, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Batista-Parra, A.; Miao, Y.; Wilson, W.D.; Wenzler, T.; Brun, R.; Boykin, D.W. Dicationic near-linear biphenyl benzimidazole derivatives as DNA-targeted antiprotozoal agents. Bioorg. Med. Chem. 2005, 13, 6718–6726. [Google Scholar] [CrossRef] [PubMed]
- El Houssine, M.; Abdelrhani, E.; Anouar, A.; Abdelilah, E.H. Synthesis of New Racemic α,α-Diaminocarboxylic Ester Derivatives. Molecules 2010, 15, 9354–9363. [Google Scholar] [CrossRef]
- Kober, R.; Steglich, W. Untersuchungen zur Reaktion von Acylaminobrommalonestern und Acylaminobromessigestern mit Trialkylphosphiten-eine einfache Synthese von 2-Amino-2-(diethoxyphosphoryl) Essigsäure Ethylester. Liebigs Ann. Chem. 1983, 4, 599–609. [Google Scholar] [CrossRef]
- Achamlale, S.; Elachqar, A.; El Hallaoui, A.; El Hajji, S.; Roumestant, ML.; Viallefont, P.H. Synthesis of α-triazolyl α-aminoacid derivatives. Amino Acids 1997, 12, 257–263. [Google Scholar] [CrossRef]
- Boukallaba, K.; Elachqar, A.; El Hallaoui, A.; Alami, A.; El Hajji, S.; Labriti, B.; Martinez, J.; Rolland, V. Synthesis of new α-heterocyclic α-aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 819–823. [Google Scholar] [CrossRef]
- Mabrouk, E.H.; Abdelrhani, E.; Abdelilah, E.H.; Anouar, A.; Soumia, E.H.; Jean, M.; Vallery, R. Synthesis of new racemic α-heterocyclic α,α-diaminoesters and α-aminoester carboxylic. Arab. J. Chem. 2010. [Google Scholar] [CrossRef]
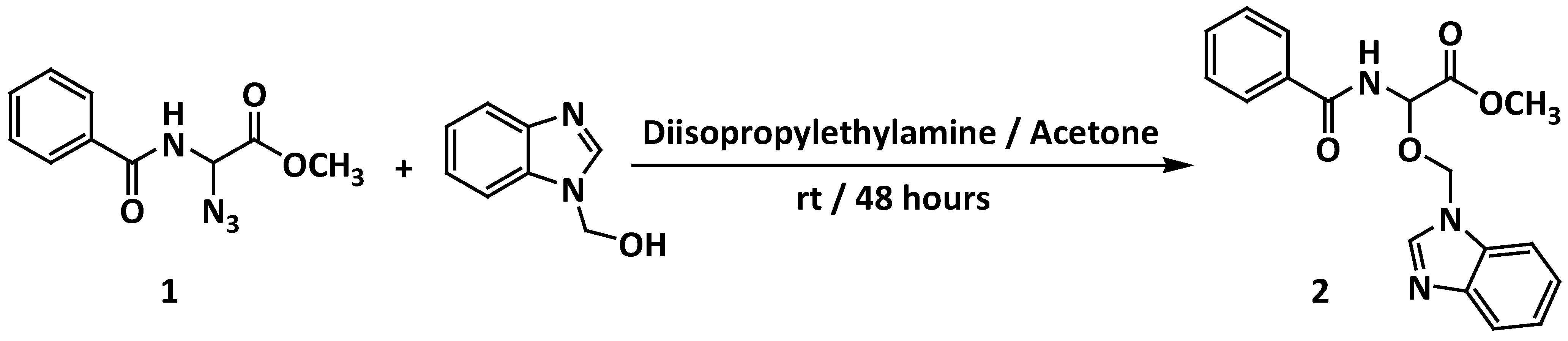
| Nu-H | Product | M.P. (°C) | Reaction Time (h) | -DCM | Et3N DCM | Et3N Acetone | DIEPA DCM | DIPEA Acetone |
|---|---|---|---|---|---|---|---|---|
| Yield (%) | Yield (%) | Yield (%) | Yield (%) | Yield (%) | ||||
| 1H-benzimidazol-1-ylmethanol | Methyl 2-benzamido-2-(1H-benzimidazol-1-ylmethoxy)acetate 2 | 116–118 | 48 | 0 | 10 | 15 | 22 | 30 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El Houssine, M.; Abdelrhani, E.; Abdelilah, E.H.; Anouar, A. Methyl 2-Benzamido-2-(1H-benzimidazol-1-ylmethoxy)acetate. Molbank 2012, 2012, M777. https://doi.org/10.3390/M777
El Houssine M, Abdelrhani E, Abdelilah EH, Anouar A. Methyl 2-Benzamido-2-(1H-benzimidazol-1-ylmethoxy)acetate. Molbank. 2012; 2012(3):M777. https://doi.org/10.3390/M777
Chicago/Turabian StyleEl Houssine, Mabrouk, Elachqar Abdelrhani, El Hallaoui Abdelilah, and Alami Anouar. 2012. "Methyl 2-Benzamido-2-(1H-benzimidazol-1-ylmethoxy)acetate" Molbank 2012, no. 3: M777. https://doi.org/10.3390/M777