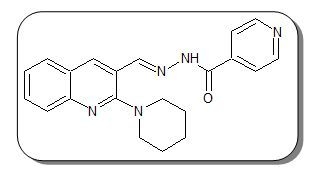
Nꞌ-{[2-(Piperidin-1-yl)quinolin-3-yl]methylene}pyridine-4-carbohydrazide
Abstract
: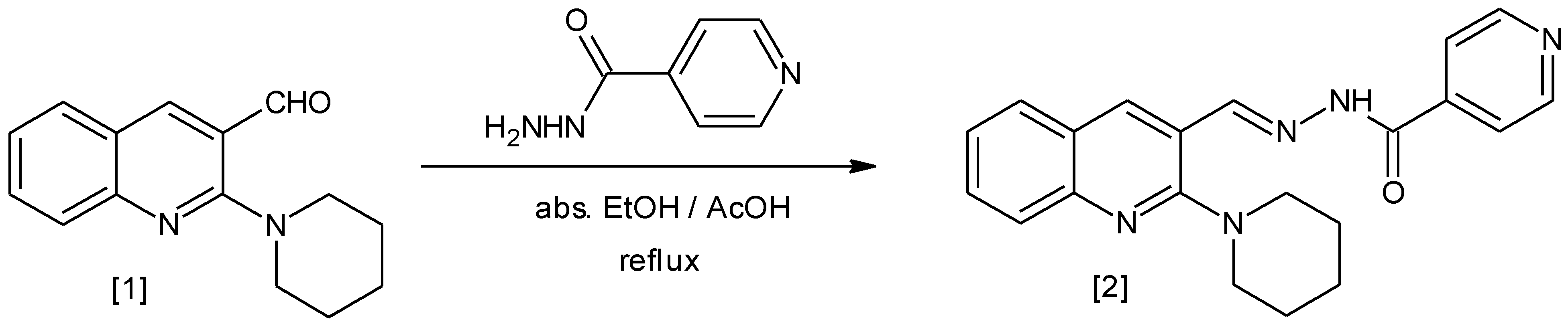
Synthesis of Nꞌ-{[2-(piperidin-1-yl)quinolin-3-yl]methylene}pyridine-4-carbohydrazide 2
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Donnell, F.O.; Smyth, T.J.P.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Wang, B.D.; Yang, Z.Y.; Li, Y.; Qin, D.D.; Li, T.R. Synthesis, crystal structure, DNA interaction and antioxidant activities of two novel water-soluble Cu2+ complexes derivated from 2-oxo-quinoline-3-carbaldehyde Schiff-bases. Eur. J. Med. Chem. 2009, 44, 4477–4484. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Ghodsi, R.; Azizi, E.; Daraie, B.; Hedayati, M.; Dadrass, O.G. Synthesis and biological evaluation of new 4-carboxyl quinoline derivatives as cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 2009, 17, 5312–5317. [Google Scholar] [CrossRef] [PubMed]
- Choy, S.; Choi, H.I.; Jung, W.H.; Kim, C.R.; Keungsikyoon; Kim, S.C.; Lee, T.G.; Koh, J.S. Synthesis of irreversible HIV-1 protease inhibitors containing sulfonamide and sulfone as amide bond isosteres. Bioorg. Med. Chem. Lett. 1997, 7, 2635–2638. [Google Scholar] [CrossRef]
- Zhou, D.; Stack, G.P.; Lo, J.; Failli, A.A.; Evrard, D.A.; Harrison, B.L. Synthesis, potency, and in vivo evaluation of 2-piperazin-1-ylquinoline analogues as dual serotonin reuptake inhibitors and serotonin 5-HT1A receptor antagonists. J. Med. Chem. 2009, 52, 4955–4959. [Google Scholar] [CrossRef] [PubMed]
- Bernotas, R.C.; Singhaus, R.R.; Kaufman, D.H.; Ullrich, J.; Fletcher, H.; Quinet, E.; Nambi, P.; Unwalla, R.; Wilhelmsson, A.; Nilsson, A.G.; Farnegardh, M.; Wrobel, J. Biarylether amide quinolines as liver X receptor agonists. Bioorg. Med. Chem. 2009, 17, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.J.; Wei, C.X.; Jia, J.H.; Zhao, L.M.; Quan, Z.S. Design and synthesis of 5-alkoxy [1,2,4]triazolo[4,3-a]quinoline derivatives with anticonvulsant activity. Eur. J. Med. Chem. 2009, 44, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Pardo, E.G. Quipazine, a new type of antidepressant agent. Psychopharmacol. (Berl.) 1971, 21, 89–100. [Google Scholar] [CrossRef]
- DiMartini, A. Isoniazid, tricyclics and the "cheese reaction". Int. Clin. Psychopharmacol. 1995, 10, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Drabu, S.; Gupta, H.; Machwal, L.; Kumar, R. Synthesis, antidepressant and antifungal evaluation of novel 2-chloro-8-methylquinoline amine derivatives. Eur. J. Med. Chem. 2011, 46, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Mali, J.R.; Bhosle, M.R.; Mahalle, S.R.; Mane, R.A. One-Pot Multicomponent Synthetic Route for New Quinolidinyl 2,4-Thiazolidinediones. Bull. Korean Chem. Soc. 2010, 31, 1859–1862. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Afzal, O.; Bawa, S.; Kumar, S.; Tonk, R.K. Nꞌ-{[2-(Piperidin-1-yl)quinolin-3-yl]methylene}pyridine-4-carbohydrazide. Molbank 2012, 2012, M748. https://doi.org/10.3390/M748
Afzal O, Bawa S, Kumar S, Tonk RK. Nꞌ-{[2-(Piperidin-1-yl)quinolin-3-yl]methylene}pyridine-4-carbohydrazide. Molbank. 2012; 2012(1):M748. https://doi.org/10.3390/M748
Chicago/Turabian StyleAfzal, Obaid, Sandhya Bawa, Suresh Kumar, and Rajiv K. Tonk. 2012. "Nꞌ-{[2-(Piperidin-1-yl)quinolin-3-yl]methylene}pyridine-4-carbohydrazide" Molbank 2012, no. 1: M748. https://doi.org/10.3390/M748