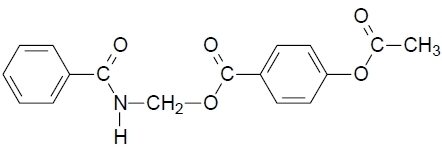
(Benzoylamino)methyl 4-Acetyloxybenzoate
Abstract
:Experimental
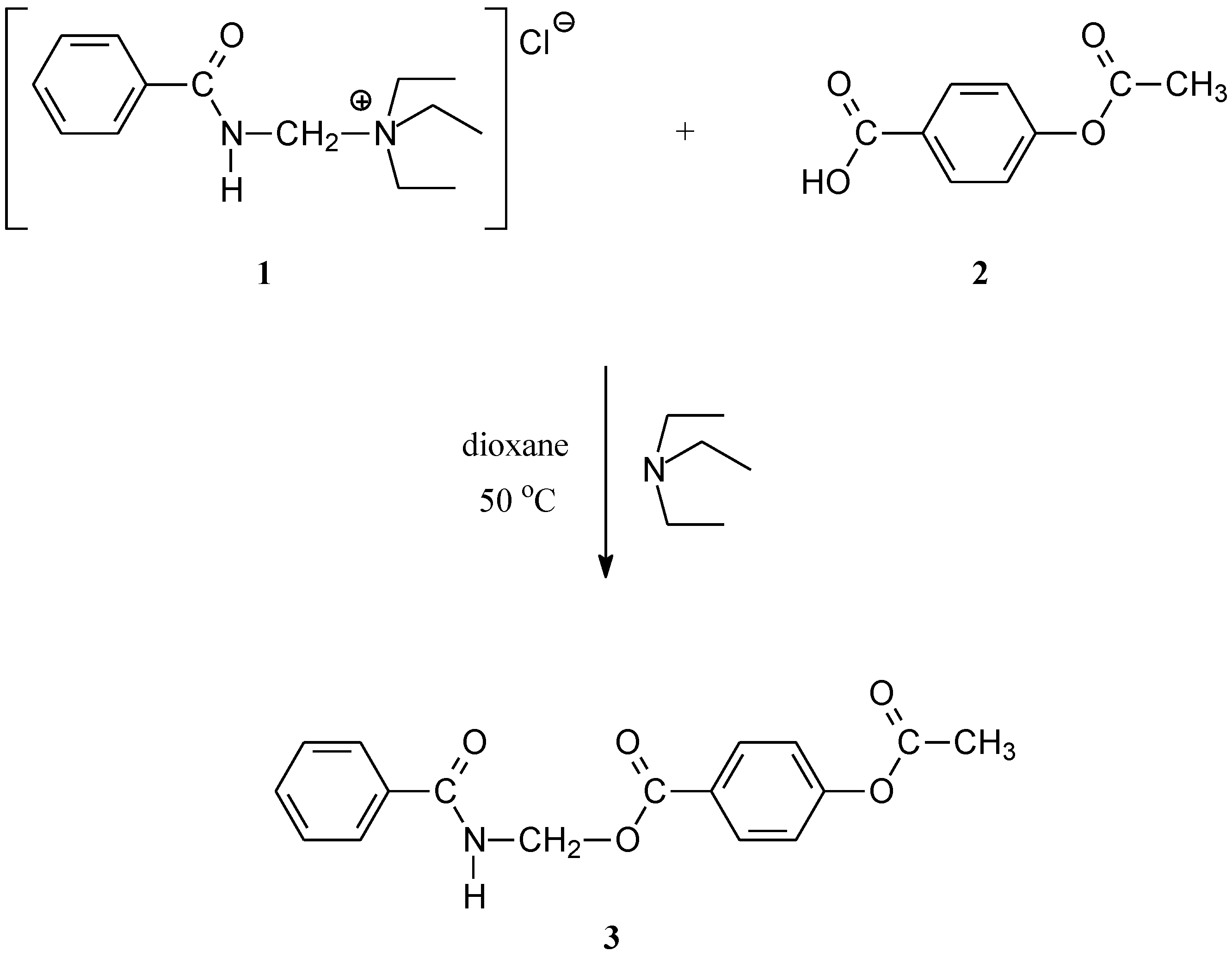
(Benzoylamino)methyl 4-acetyloxybenzoate (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Popovski, E.; Mladenovska, K. (Benzoylamino)methyl 4-hydroxybenzoate. Molbank 2010, 2010, M658. [Google Scholar] [CrossRef]
- Popovski, E.; Mladenovska, K.; Panovska, A.P. (Benzoylamino)methyl 4-[(Benzoylamino)methoxy]benzoate. Molbank 2011, 2011, M711. [Google Scholar] [CrossRef]
- Popovski, E.; Mladenovska, K. Panovska, A.P. Methyl 4-[(Benzoylamino)methoxy]benzoate. Molbank 2011, 2011, M712. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acids. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Simple method for benzamidomethylation of phenols in water solution. Synth. Commun. 1999, 29, 3451–3458. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Popovski, E.; Mladenovska, K. (Benzoylamino)methyl 4-Acetyloxybenzoate. Molbank 2011, 2011, M715. https://doi.org/10.3390/M715
Popovski E, Mladenovska K. (Benzoylamino)methyl 4-Acetyloxybenzoate. Molbank. 2011; 2011(1):M715. https://doi.org/10.3390/M715
Chicago/Turabian StylePopovski, Emil, and Kristina Mladenovska. 2011. "(Benzoylamino)methyl 4-Acetyloxybenzoate" Molbank 2011, no. 1: M715. https://doi.org/10.3390/M715