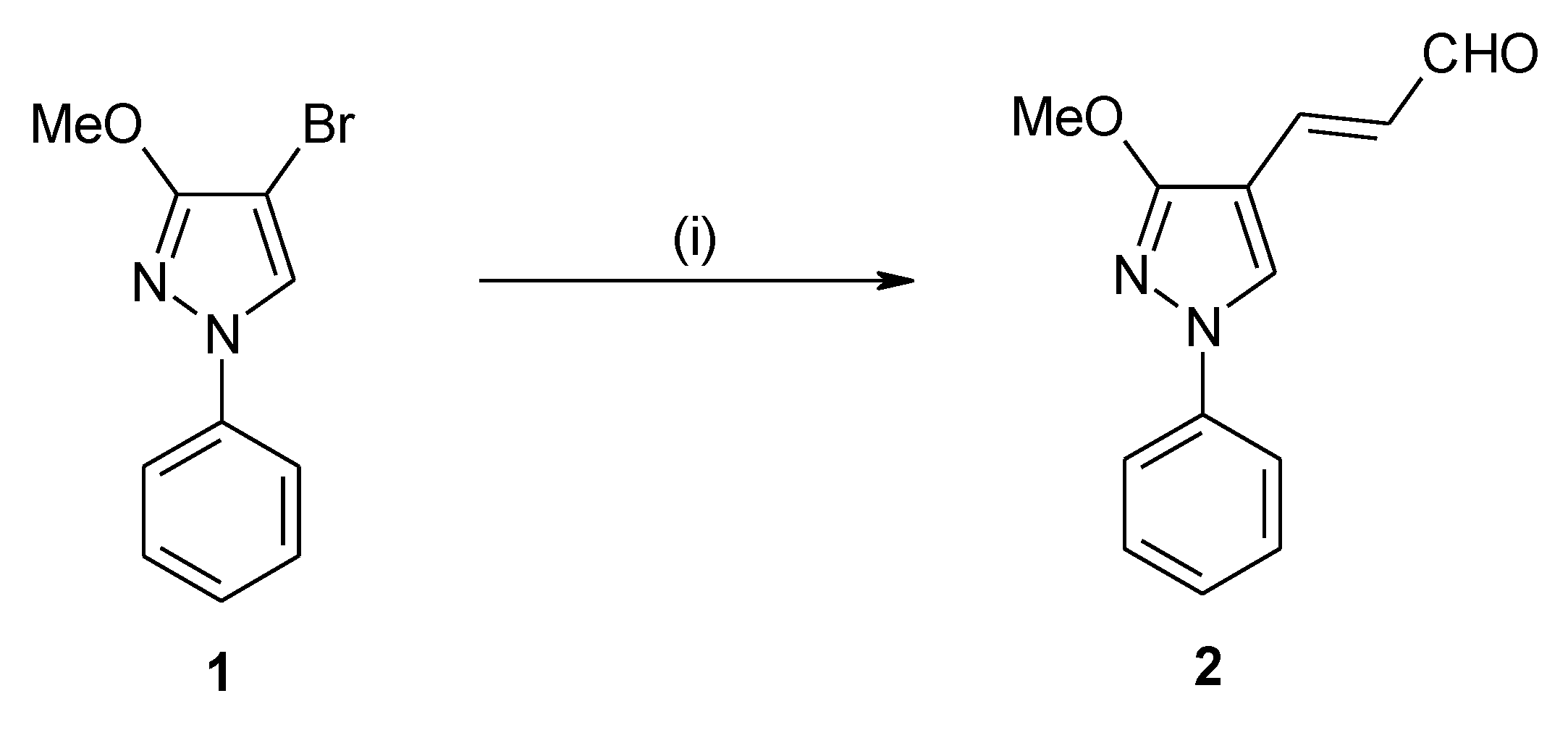
(2E)-3-(3-Methoxy-1-phenyl-1H-pyrazol-4-yl)-2-propenal
Abstract
:Experimental
(2E)-3-(3-Methoxy-1-phenyl-1H-pyrazol-4-yl)-2-propenal (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Juárez, E.; García, A.; Hommer, H.; Salas, M.; Gordillo, B. Stereoselective synthesis and conformational analysis of aromatic C-thionucleosides. Heteroatom Chem. 2006, 17, 289–298. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, X.X.; Rao, Q.Q.; Gong, L.Z. Organocatalytic asymmetric three-component cyclization of cinnamaldehydes and primary amines with 1,3-dicarbonyl compounds: Straightforward access to enantiomerically enriched dihydropyridines. Angew. Chem. Int. Ed. 2008, 47, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.C.; Liu, K.L.; Tsai, C.W.; Liao, J.H. Proline-mediated dimerization of cinnamdehydes via 1,3-dipolar cycloaddition reaction with azomethine ylides. A rapid access to highly functionalized hexahydro-1H-pyrrolizine. Tetrahedron Lett. 2008, 49, 5480–5483. [Google Scholar] [CrossRef]
- Jiang, J.; Qing, J.; Gong, L.Z. Asymmetric synthesis of 3-amino-δ-lactams and benzo[a]quinolizines by catalytic cyclization reactions involving azlactones. Chem. Eur. J. 2009, 15, 7031–7034. [Google Scholar] [CrossRef] [PubMed]
- Shachkus, A.A.; Degutis, Y.A. Synthesis of 8-phenyl-10H-pyrido[1,2-a]indole salts from 2,3,3-trimethyl-3H-indole chlorides with cinnamaldehyde. Chem. Heterocycl. Comp. 1987, 23, 401–403. [Google Scholar] [CrossRef]
- Battistuzzi, G.; Cacchi, S.; Fabrizi, G. An efficient palladium-catalyzed synthesis of cinnamaldehydes from acrolein diethyl acetal and aryl iodides and bromides. Org. Lett. 2003, 5, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Noël, S.; Luo, C.; Pinel, C.; Djakovitch, L. Efficient heterogeneously palladium-catalysed Heck arylation of acrolein diethyl acetal. Synthesis of cinnamaldehydes or 3-arylpropionic esters. Adv. Synth. Catal. 2007, 349, 1128–1140. [Google Scholar] [CrossRef]
- Alacid, E.; Najera, C. Acrolein diethyl acetal: A three-carbon homolating reagent for the synthesis of β-arylpropanoates and cinnamaldehydes by Heck reaction catalyzed by a Kaiser oxime resin derived palladacycle. Eur. J. Org. Chem. 2008, 18, 3102–3106. [Google Scholar] [CrossRef]
- Berthiol, F.; Doucet, H.; Santelli, M. Direct synthesis of cinnamaldehyde derivatives by reaction of aryl bromides with 3,3-diacetoxypropene catalyzed by a palladium-tetraphosphine complex. Catalysis Letters 2005, 102, 281–284. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Vilkauskaitė, G.; Eller, G.A.; Holzer, W.; Šačkus, A. Pd-catalyzed cross-coupling reactions of halogenated 1-phenylpyrazol-3-ols and related triflates. Tetrahedron 2009, 65, 7817–7824. [Google Scholar] [CrossRef]
- Holzer, W.; Krca, I. New 1-substituted 4-cinnamoyl-5-hydroxypyrazoles and precursors thereof: Synthesis, ring closure reactions and NMR-spectroscopic investigations. Heterocycles 2003, 60, 2323–2342. [Google Scholar] [CrossRef]
- Heinisch, G.; Holzer, W.; Huber, T. Ein effizienter Zugang zu aryl- oder benzyl-4-pyrazolylketonen und carbinolen. Arch. Pharm. (Weinheim) 1987, 320, 1267–1272. [Google Scholar] [CrossRef]
- Heinisch, G.; Holzer, W.; Obala, C. Beiträge zur Chemie von Pyrazolylalkinen. Monatsh. Chem. 1988, 119, 253–262. [Google Scholar] [CrossRef]
- Koenig, H.; Goetz, N.; Klein, U.; Eller, K. Process for producing N-substituted 3-hydroxypyrazoles. Chem. Abstr. 1997, 126. 199566, WO 9703969. [Google Scholar]
- Kleizienė, N.; Arbačiauskienė, E.; Holzer, W.; Šačkus, A. 4-Bromo-3-methoxy-1-phenyl-1H-pyrazole. Molbank 2009, M639. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kleizienė, N.; Arbačiauskienė, E.; Holzer, W.; Šačkus, A. (2E)-3-(3-Methoxy-1-phenyl-1H-pyrazol-4-yl)-2-propenal. Molbank 2009, 2009, M644. https://doi.org/10.3390/M644
Kleizienė N, Arbačiauskienė E, Holzer W, Šačkus A. (2E)-3-(3-Methoxy-1-phenyl-1H-pyrazol-4-yl)-2-propenal. Molbank. 2009; 2009(4):M644. https://doi.org/10.3390/M644
Chicago/Turabian StyleKleizienė, Neringa, Eglė Arbačiauskienė, Wolfgang Holzer, and Algirdas Šačkus. 2009. "(2E)-3-(3-Methoxy-1-phenyl-1H-pyrazol-4-yl)-2-propenal" Molbank 2009, no. 4: M644. https://doi.org/10.3390/M644



