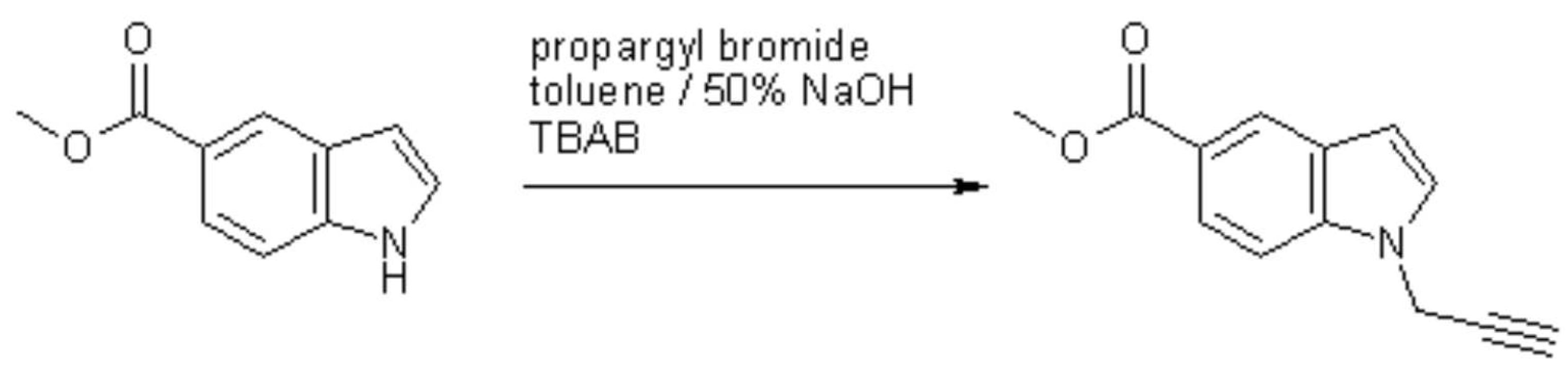
Methyl 1-prop-2-yn-1-yl-1H-indole-5-carboxylate
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Broggini, G.; Bruché, L.; Zecchi, G.; Pilati, T. 1,3-Dipolar cycloadditions to nitrogen-substituted allenes. J. Chem. Soc., Perkin Trans. 1 1990, 533–539. [Google Scholar] [CrossRef]
- For recent examples of alkyne/allene rearrangements of N-propargylindoles, see: Haider, N.; Käferböck, J. Intramolecular [4+2] cycloaddition reactions of indolylalkylpyridazines: synthesis of annulated carbazoles. Tetrahedron 2004, 60, 6495–6507. [Google Scholar] Abbiati, G.; Canevari, V.; Caimi, S.; Rossi, E. Domino addition/annulation of delta-alkynylaldehydes and oxygen nucleophiles. A new entry to [1,4]oxazino[4,3-a]indoles. Tetrahedron Lett. 2005, 46, 7117–7120. [Google Scholar]
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Haider, N.; Kabicher, T. Methyl 1-prop-2-yn-1-yl-1H-indole-5-carboxylate. Molbank 2007, 2007, M560. https://doi.org/10.3390/M560
Haider N, Kabicher T. Methyl 1-prop-2-yn-1-yl-1H-indole-5-carboxylate. Molbank. 2007; 2007(5):M560. https://doi.org/10.3390/M560
Chicago/Turabian StyleHaider, Norbert, and Tamara Kabicher. 2007. "Methyl 1-prop-2-yn-1-yl-1H-indole-5-carboxylate" Molbank 2007, no. 5: M560. https://doi.org/10.3390/M560




