Myosin Assembly, Maintenance and Degradation in Muscle: Role of the Chaperone UNC-45 in Myosin Thick Filament Dynamics
Abstract
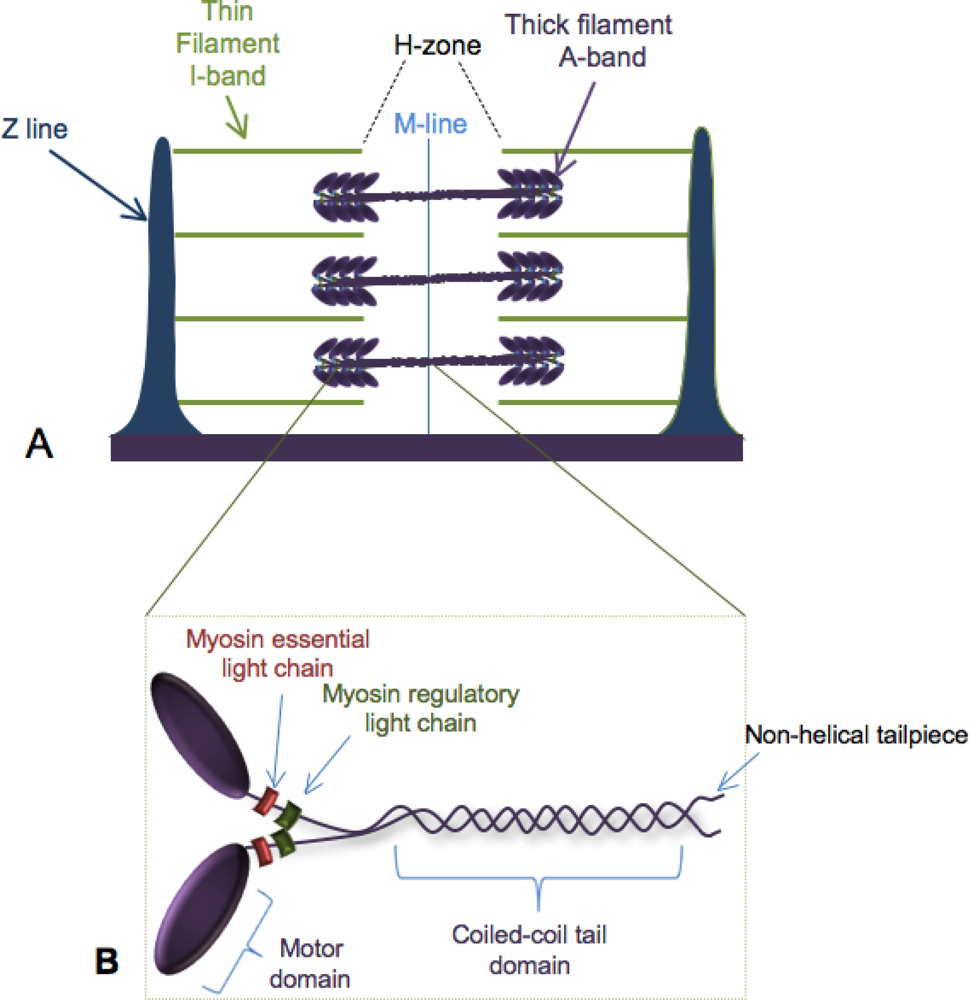
:1. Building the Sarcomere
2. Myosin Folding
2.1. Role of unc-45 in myosin folding
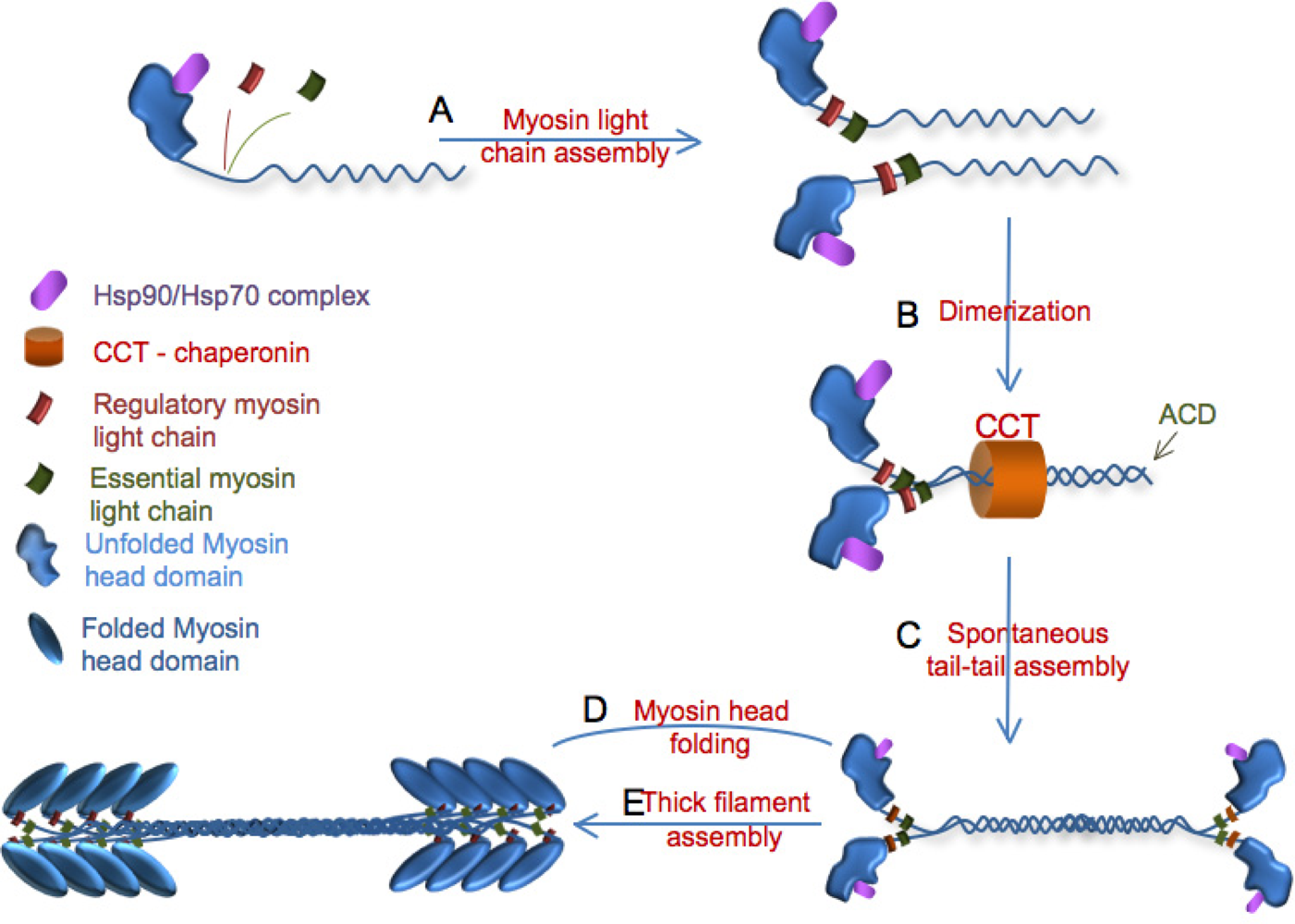
3. Myosin Assembly
3.1. Role of UNC-45 in myofibril assembly
4. Maintenance of the Thick Filament
5. Turnover of the Thick Filament Components
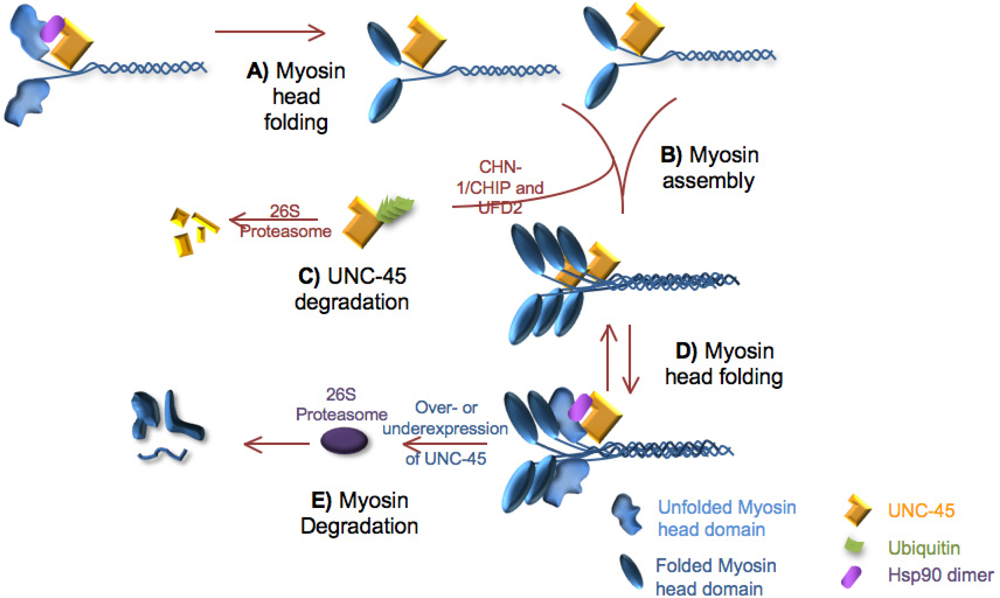
5.1. UNC-45 and myosin turnover
6. Human diseases of thick filament organization
7. Conclusions
References
- Berg, JS; Powell, BC; Cheney, RE. A millennial myosin census. Mol. Biol. Cell 2001, 12, 780–794. [Google Scholar]
- Srikakulam, R; Winkelmann, DA. Chaperone-mediated folding and assembly of myosin in striated muscle. J. Cell Sci 2004, 117, 641–52. [Google Scholar]
- Winkelmann, DA; Baker, TS; Rayment, I. Three-dimensional structure of myosin subfragment-1 from electron microscopy of sectioned crystals. J. Cell Biol 1991, 114, 701–713. [Google Scholar]
- Rayment, I; Holden, HM. The three-dimensional structure of a molecular motor. Trends Biochem. Sci 1994, 19, 129–134. [Google Scholar]
- Rayment, I. Kinesin and myosin: Molecular motors with similar engines. Structure 1996, 4, 501–504. [Google Scholar]
- Barral, JM; Epstein, HM. Protein machines and self assembly in muscle organization. Bioessays 1999, 21, 813–823. [Google Scholar]
- Srikakulam, R; Winkelmann, DA. Myosin II folding is mediated by a molecular chaperonin. J. Biol. Chem 1999, 274, 27265–27273. [Google Scholar]
- Hutagalung, AH; Landsverk, ML; Price, MG; Epstein, HF. The UCS family of myosin chaperones. J. Cell Sci 2002, 115, 3983–3990. [Google Scholar]
- Chow, D; Srikakulam, R; Chen, Y; Winkelmann, DA. Folding of the striated muscle myosin motor domain. J. Biol. Chem 2002, 277, 36799–36807. [Google Scholar]
- Epstein, HF; Thomson, JN. Temperature-sensitive mutation affecting myofilament assembly in C. elegans. Nature 1974, 250, 579–580. [Google Scholar]
- Venolia, L; Waterston, RH. The unc-45 gene of C. elegans is an essential muscle-affecting gene with maternal expression. Genetics 1990, 126, 345–354. [Google Scholar]
- Barral, JM; Bauer, CC; Ortiz, I; Epstein, HF. Unc-45 mutations in Caenorhabditis elegans implicates a CRO1/She4p-like domain in myosin assembly. J. Cell Biol 1998, 143, 1215–1225. [Google Scholar]
- Ao, W; Pilgrim, D. Caenorhabditis elegans UNC-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A. J. Cell Biol 2000, 148, 375–384. [Google Scholar]
- Price, MG; Landsverk, ML; Barral, JM; Epstein, HF. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J. Cell Sci 2002, 115, 4013–4023. [Google Scholar]
- Etheridge, L; Diiorio, P; Sagerström, CG. A zebrafish unc-45-related gene expressed during muscle development. Dev. Dyn 2002, 224, 457–460. [Google Scholar]
- Wohlgemuth, SL; Crawford, B; Pilgrim, DB. The myosin co-chaperone UNC-45 is required for skeletal and cardiac muscle function in zebrafish. Dev. Biol 2007, 303, 483–492. [Google Scholar]
- Etard, C; Behra, M; Fischer, N; Hutcheson, D; Geisler, R; Strähle, U. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev. Biol 2007, 308, 133–143. [Google Scholar]
- Chadli, A; Graham, JD; Abel, MG; Jackson, TA; Gordon, DF; Wood, WM; Felts, SJ; Horwitz, KB; Toft, D. GCUNC-45 is a novel regulator for the progesterone receptor/hsp90 chaperoning pathway. Mol. Cell Biol 2006, 26, 1722–1730. [Google Scholar]
- Chadli, A; Bruinsma, ES; Stensgard, B; Toft, D. Hsp90 cochaperone interactions reveals a novel mechanism for TPR protein recognition. Biochem 2008, 47, 2850–2857. [Google Scholar]
- Chadli, A; Felts, SJ; Toft, D. GCUNC45 is the first Hsp90 cochaperone to show α/β isoform specificity. J. Biol. Chem 2008, 283, 9509–9512. [Google Scholar]
- Bazzaro, M; Santillan, A; Lin, Z; Tang, T; Lee, MK; Bristow, RE; Shih, IM; Roden, RB. Myosin II Co-Chaperone General Cell UNC-45 Overexpression Is Associated with Ovarian Cancer, Rapid Proliferation, and Motility. Am. J. Pathol 2007, 171, 1640–1649. [Google Scholar]
- Anderson, MJ; Pham, VN; Vogel, AM; Weinstein, BM; Roman, BL. Loss of unc-45a precipitates arteriovenous shunting in the aortic arches. Dev. Biol 2008, 318, 258–267. [Google Scholar]
- Liu, L; Srikakulam, R; Winkelmann, DA. Unc45 activates Hsp90 dependent folding of the myosin motor domain. J. Biol. Chem 2008, 283, 13185–13193. [Google Scholar]
- Barral, JM; Hutagalung, AH; Brinker, A; Hartl, FU; Epstein, HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 2002, 295, 669–671. [Google Scholar]
- Srikakulam, R; Liu, L; Winkelmann, DA. Unc45b forms a cytosolic complex with Hsp90 and targets the unfolded myosin motor domain. PLoS ONE 2008, 3, e2137. [Google Scholar]
- Kimmins, S; MacRae, TH. Maturation of steroid receptors: An example of functional cooperation among molecular chaperones and their associated proteins. Cell Stress Chaperon 2000, 5, 76–86. [Google Scholar]
- Pearl, LH; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Ann. Rev. Biochem 2006, 75, 271–294. [Google Scholar]
- Hawkins, TA; Haramis, AP; Etard, C; Prodromou, C; Vaughan, CK; Ashworth, R; Ray, S; Behra, M; Holder, N; Talbot, WS; Pearl, LH; Strähle, U; Wilson, SW. The ATPase-dependent chaperoning activity of Hsp90a regulates thick filament formation and integration during skeletal muscle myofibrillogenesis. Development 2008, 135, 1147–1156. [Google Scholar]
- Du, SJ; Li, H; Bian, Y; Zhong, Y. Heat-shock protein 90alpha1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc. Nat. Acad. Sci. USA 2008, 105, 554–559. [Google Scholar]
- Valpuesta, JM; Martín-Benito, J; Gómez-Puertas, P; Carrascosa, JL; Willison, KR. Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett 2002, 529, 11–16. [Google Scholar]
- Sohn, RL; Vikstrom, KL; Strauss, M; Cohen, C; Szent-Gyorgyi, AG; Leinwand, LA. A 29 residue region of the sarcomeric myosin rod is necessary for filament formation. J. Mol. Biol 1997, 266, 317–330. [Google Scholar]
- Arrizubieta, MJ; Bandman, E. Regulation of alpha-helical coiled-coil dimerization in chicken skeletal muscle light meromyosin. J. Biol. Chem 1999, 274, 13847–13853. [Google Scholar]
- Eddinger, TJ; Meer, DP. Myosin II isoforms in smooth muscle: heterogeneity and function. Am. J. Physiol. Cell Physiol 2007, 293, C493–508. [Google Scholar]
- Schachat, FH; Harris, HE; Epstein, HF. Two homogeneous myosins in body-wall muscle of Caenorhabditis elegans. Cell 1977, 10, 721–728. [Google Scholar]
- Miller, DM, 3rd; Ortiz, I; Berliner, GC; Epstein, HF. Differential localization of two myosins within nematode thick filaments. Cell 1983, 34, 477–490. [Google Scholar]
- Hoppe, P; Andrews, RC; Parikh, R. Differential requirement for the non-helical tailpiece and the C-terminus of the myosin rod in Caenorhabditis elegans muscle. Mol. Biol. Cell 2003, 14, 1677–1690. [Google Scholar]
- Weinert, S; Bergmann, N; Luo, X; Erdmann, B; Gotthardt, M. M line-deficient titin causes cardiac lethality through impaired maturation of the sarcomere. J. Cell Biol 2006, 173, 559–567. [Google Scholar]
- Sanger, JW; Kang, S; Siebrands, CC; Freeman, N; Du, A; Wang, J; Stout, AL; Sanger, JM. How to build a myofibril. J. Muscle Res. Cell Motil 2005, 26, 343–354. [Google Scholar]
- Janiesch, PC; Kim, J; Mouysset, J; Barikbin, R; Lochmüller, H; Cassata, G; Krause, S; Hoppe, T. The ubiquitin-selective chaperone CDC-48/p97 links myosin assembly to human myopathy. Nat. Cell Biol 2007, 9, 379–390. [Google Scholar]
- Hoppe, T; Cassata, G; Barral, JM; Springer, W; Hutagalung, AH; Epstein, HF; Baumeister, R. Regulation of the myosin-directed chaperone UNC-45 by the novel E3/E4-multiubiquitylation complex in C. elegans. Cell 2004, 118, 337–349. [Google Scholar]
- Kim, J; Löwe, T; Hoppe, T. Protein quality control gets muscle into shape. Trends Cell Biol 2008, 18, 264–272. [Google Scholar]
- Markov, DI; Pivovarova, AV; Chernik, IS; Gusev, NB; Levitsky, DI. Small heat shock protein Hsp27 protects myosin S1 from heat-induced aggregation, but not from thermal denaturation and ATPase inactivation. FEBS Lett 2008, 582, 1407–1412. [Google Scholar]
- Johnson, CP; Tang, HY; Carag, C; Speicher, DW; Discher, DE. Forced unfolding of proteins within cells. Science 2007, 317, 663–666. [Google Scholar]
- Golenhofen, N; Htun, P; Ness, W; Koob, R; Schaper, W; Drenckhahn, D. Binding of the stress protein alphaB-crystallin to cardiac microfibrils correlates with the degree of myocardial damage during ischemia/reperfusion in vivo. J. Mol. Cell. Cardiol 1999, 31, 569–580. [Google Scholar]
- van de Klundert, FA; Smulders, RH; Gijsen, ML; Linder, RA; Jaenicke, R; Carver, JA; de Jong, WW. The mammalian small heat-shock protein Hsp20 forms dimmers and is a poor chaperone. Eur. J. Biochem 1998, 258, 1014–1021. [Google Scholar]
- Barbato, R; Menabo, R; Dainese, P; Carafoli, E; Schiaffino, S; DiLisa, F. Binding of cytosolic proteins to myofibrils in ischemic rat hearts. Circ. Res 1996, 78, 821–828. [Google Scholar]
- Inagaki, N; Hayashi, T; Arimura, T; Koga, Y; Takahashi, M; Shibata, H; Teraoka, K; Chikamori, T; Yamashina, A; Kimura, A. Alpha B-crystallin mutation in diluted cardiomyopathy. Biochem. Biophys. Res. Comm 2006, 342, 379–386. [Google Scholar]
- Etard, C; Roostalu, U; Strahle, U. Shuttling of the chaperones Unc45b and Hsp90a between the A band and the Z line of the myofibril. J. Cell Biol 2008, 180, 1163–1175. [Google Scholar]
- Kachur, TM; Audhya, A; Pilgrim, DB. UNC-45 is required for NMY-2 contractile function in early embryonic polarity establishment and germline cellularization in C. elegans. Dev. Biol 2008, 314, 287–299. [Google Scholar]
- Lecker, SH; Solomon, V; Mitch, WE; Goldberg, AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J. Nutri 1999, 129, 227S–237S. [Google Scholar]
- Wang, X; Robbins, J. Heart failure and protein quality control. Circ. Res 2006, 99, 1315–1328. [Google Scholar]
- Acharyya, S; Ladner, KJ; Nelsen, LL; Damrauer, J; Reiser, PJ; Swoap, S; Guttridge, DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest 2004, 114, 370–378. [Google Scholar]
- Soloman, V; Goldberg, AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J. Biol. Chem 1996, 271, 26690–26697. [Google Scholar]
- Landsverk, ML; Li, S; Hutagalung, AH; Najafov, A; Hoppe, T; Barral, JM; Epstein, HF. The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J. Cell Biol 2007, 177, 205–210. [Google Scholar]
- Brown, DD; Christine, KS; Showell, C; Conlon, FL. Small heat shock protein Hsp27 is required for proper heart tube formation. Genesis 2007, 45, 667–678. [Google Scholar]
- Bönnemann, CG; Laing, NG. Myopathies resulting from mutations in sarcomeric proteins. Curr. Opin. Neurol 2004, 17, 529–537. [Google Scholar]
- Tajsharghi, H; Thornell, LE; Lindberg, C; Lindvall, B; Henriksson, KG; Oldfors, A. Myosin storage myopathy associated with a heterozygous missense mutation in MYH7. Ann. Neurol 2003, 54, 494–500. [Google Scholar]
- Bohlega, S; Abu-Amero, SN; Wakil, SM; Carroll, P; Al-Amr, R; Lach, B; Al-Sayed, Y; Cupler, EJ; Meyer, BF. Mutation of the slow myosin heavy chain rod domain underlies hyaline body myopathy. Neurology 2004, 62, 1518–1521. [Google Scholar]
- Darin, N; Tajsharghi, H; Ostman-Smith, I; Gilljam, T; Oldfors, A. New skeletal myopathy and cardiomyopathy associated with a missense mutation in MYH7. Neurology 2007, 68, 2041–2042. [Google Scholar]
- Doran, P; Gannon, J; O’Connell, K; Ohlendieck, K. Aging skeletal muscle shows a drastic increase in the small heat shock proteins alphaB-crystallin/HspB5 and cvHsp/HspB7. Eur. J. Cell Biol 2007, 86, 629–640. [Google Scholar]
- Bodine, SC; Latres, E; Baumhueter, S; Lai, VK; Nunez, L; Clarke, BA; Poueymirou, WT; Panaro, FJ; Na, E; Dharmarajan, K; Pan, ZQ; Valenzuela, DM; DeChiara, TM; Stitt, TN; Yancopoulos, GD; Glass, DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar]
© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kachur, T.M.; Pilgrim, D.B. Myosin Assembly, Maintenance and Degradation in Muscle: Role of the Chaperone UNC-45 in Myosin Thick Filament Dynamics. Int. J. Mol. Sci. 2008, 9, 1863-1875. https://doi.org/10.3390/ijms9091863
Kachur TM, Pilgrim DB. Myosin Assembly, Maintenance and Degradation in Muscle: Role of the Chaperone UNC-45 in Myosin Thick Filament Dynamics. International Journal of Molecular Sciences. 2008; 9(9):1863-1875. https://doi.org/10.3390/ijms9091863
Chicago/Turabian StyleKachur, Torah M., and David B. Pilgrim. 2008. "Myosin Assembly, Maintenance and Degradation in Muscle: Role of the Chaperone UNC-45 in Myosin Thick Filament Dynamics" International Journal of Molecular Sciences 9, no. 9: 1863-1875. https://doi.org/10.3390/ijms9091863



