Microwave-Assisted Esterification of N-Acetyl-L-Phenylalanine Using Modified Mukaiyama’s Reagents: A New Approach Involving Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of solvents and bases
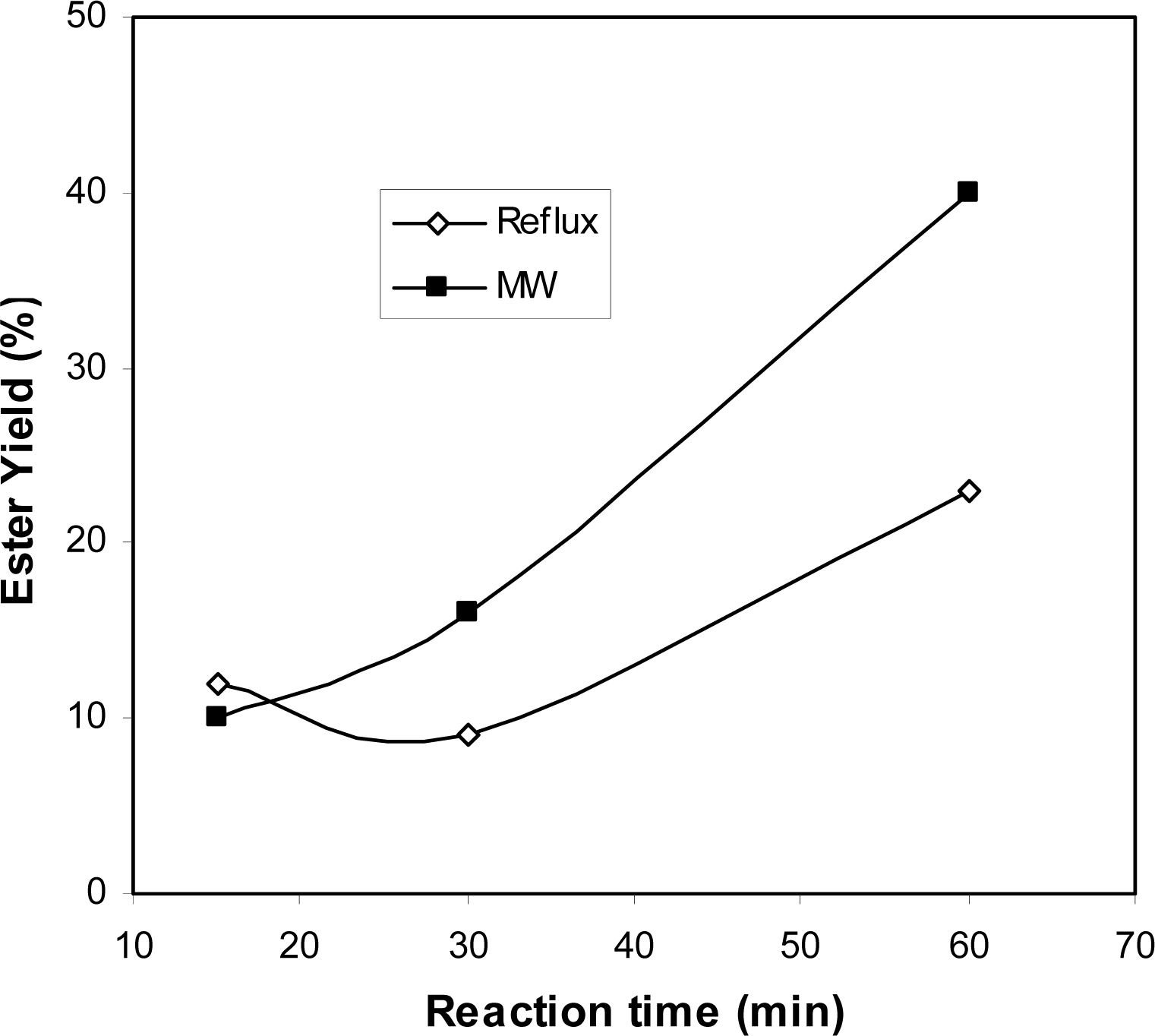
2.2 Comparison of microwave and conventional heating
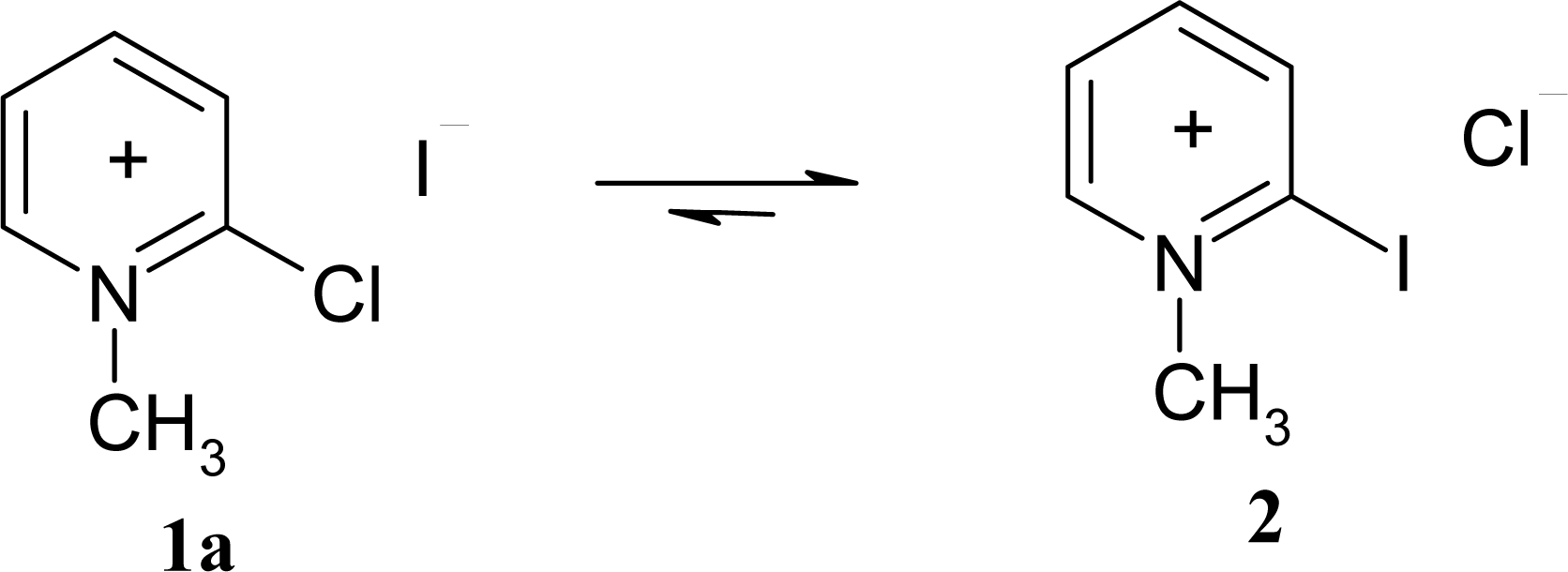
2.3 Modification of Mukaiyama's reagent
2.4 Racemization of amino acid ester
3. Experimental Section
3.1 Materials
3.2 Synthesis of modified Mukaiyama's reagents (2-Chloro-1-methylpyridinium acetate ([2- ClMePy][CH3COO]), 2-Chloro-1-methylpyridinium trifluoroacetate ([2-ClMePy][CF3COO]), and 2- Chloro-1-methylpyridinium tetrafluoroborate ([2-ClMePy][BF4]))
3.3 Synthesis of 2-Chloro-1-methylpyridinium ethyl sulfate ([2-ClMePy][EtSO4])
3.4 Synthesis of 2-Chloro-1-methylpyridinium bis(trifluoromethane)sulfonimide ([2-ClMePy][Tf2N])
3.5 General procedure for esterification of amino acid
Acknowledgements
- §Calculated from the specific rotation of 15.4 with 99.1% ee at the same condition.
References and Notes
- Bodanszky, M; Klausner, YS; Ondetti, MA. Peptide Synthesis, 2nd ed; Wiley: New York, 1976; p. 51. [Google Scholar]
- Greenstein, JP; Winitz, M. Chemistry of the Amino Acids; Krieger Publishing Co.: Malabar, 1984; Vol. 2, Chapter 10. [Google Scholar]
- Sheldon, RA. Chiral Technology: Industrial Synthesis of Optically Active Compounds; Marcel Dekker: New York, 1993. [Google Scholar]
- Kita, H; Sasaki, S; Tanaka, K; Okamoto, K; Yamamoto, M. Esterification of carboxylic acid with ethanol accompanied by pervaporation. Chem Lett 1988, 2025–2028. [Google Scholar]
- Arai, I; Muramatsu, I. A simple and convenient method for esterification of tryptophan and other amino acids. J Org Chem 1983, 48, 121–123. [Google Scholar]
- Miller, HK; Waelsch, H. Benzyl esters of amino acids. J Am Chem Soc 1952, 74, 1092–1093. [Google Scholar]
- Wegman, MA; Elzinga, JM; Neeleman, E; van Rantwijk, F; Sheldon, RA. Salt-free esterification of α-amino acids catalysed by zeolite H-USY. Green Chem 2001, 3, 61–64. [Google Scholar]
- Bodanszky, M; Bodanszky, A. The practice of peptide synthesis; Springer-Verlag: Berlin, 1984. [Google Scholar]
- Jones, JB; Niemann, C. A further comparison of the behavior of analogous aromatic and hydroaromatic substrates of α-chymotrypsin. Biochemistry 1963, 2, 498–500. [Google Scholar]
- Wang, S-S; Gisin, BF; Winter, DP; Makofske, R; Kulesha, ID; Tzougraki, C; Meienhofer, J. Facile synthesis of amino acid and peptide esters under mild conditions via cesium salts. J Org Chem 1977, 42, 1286–1290. [Google Scholar]
- Shoda, S; Mukaiyama, T. Cesium fluoride-promoted synthesis of carboxylic acid derivatives using 2-fluoropyridinium salt. Chem Lett 1980, 391–392. [Google Scholar]
- Sato, T; Otera, J; Nozaki, H. Cesium fluoride-promoted esterification of carboxylic acids. A practical alternative to the diazomethane method and direct conversion of organotin carboxylates. J Org Chem 1992, 57, 2166–2169. [Google Scholar]
- Biondini, D; Brinchi, L; Germani, R; Savelli, G. An effective chemoselective esterification of hydroxybenzoic acids in ionic liquid promoted by KF. Lett Org Chem 2006, 3, 207–211. [Google Scholar]
- Mukaiyama, T; Oohashi, Y; Fukumo, K. A new method for the esterification of carboxylic acids with various alcohols by using di-2-thienyl carbonate, a new coupling reagent. Chem Lett 2004, 33, 552–553. [Google Scholar]
- Mukaiyama, T. New synthetic reactions based on the onium salts of azaarenes. Angew Chem Int Ed Engl 1979, 18, 707–721. [Google Scholar]
- Mukaiyama, T; Usui, M; Shimada, E; Saigo, K. A convenient method for the synthesis of carboxylic esters. Chem Lett 1975, 1045–1048. [Google Scholar]
- Saigo, K; Usui, M; Kikuchi, K; Shimada, E; Mukaiyama, T. New method for the preparation of carboxylic esters. Bull Chem Soc Jpn 1977, 50, 1863–1866. [Google Scholar]
- Crosignani, S; Gonzalez, J; Swinnen, D. Polymer-supported mukaiyama reagent: A useful coupling reagent for the synthesis of esters and amides. Org Lett 2004, 6, 4579–4582. [Google Scholar]
- Donati, D; Morelli, C; Taddei, M. A rapid microwave-assisted esterification utilizing the Mukaiyama supported reagent. Tetrahedron Lett 2005, 46, 2817–2819. [Google Scholar]
- Convers, E; Tye, H; Whittaker, M. Preparation and evaluation of a polymer-supported Mukaiyama reagent. Tetrahedron Lett 2004, 45, 3401–3404. [Google Scholar]
- Donati, D; Morelli, C; Porcheddu, A; Taddei, M. A new polymer-supported reagent for the synthesis of β-lactams in solution. J Org Chem 2004, 69, 9316–9318. [Google Scholar]
- Gordon, CM. New developments in catalysis using ionic liquids. Appl Cat A: General 2001, 222, 101–117. [Google Scholar]
- Houlton, S. Ionic liquids: the route to cleaner and more efficient fine chemical synthesis? Chem Week 2004, s10–s11. [Google Scholar]
- Seddon, KR. Ionic liquids for clean technology. J Chem Technol Biotechnol 1997, 68, 351–356. [Google Scholar]
- Welton, T. Room-temperature ionic liquids - solvents for synthesis and catalysis. Chem Rev 1999, 99, 2071–2083. [Google Scholar]
- Zhao, H; Malhotra, SV. Applications of ionic liquids in organic synthesis. Aldrichimica Acta 2002, 35, 75–83. [Google Scholar]
- Earle, M; Forestier, A; Olivier-Bourbigou, H; Wasserscheid, P. Ionic Liquids in Synthesis; Wasserscheid, P, Welton, T, Eds.; Wiley-VCH Verlag: Weinheim, 2003; pp. 174–288. [Google Scholar]
- Jain, N; Kumar, A; Chauhan, S; Chauhan, SMS. Chemical and biochemical transformations in ionic liquids. Tetrahedron 2005, 61, 1015–1060. [Google Scholar]
- Bradlow, HL; Vanderwerf, CA. Exchange reactions of α-halogenated pyridines. J Org Chem 1951, 16, 1143–1152. [Google Scholar]
- Oh, SH; Cortez, GS; Romo, D. Asymmetric synthesis of bicyclic β-lactones via the intramolecular, nucleophile-catalyzed aldol lactonization: Improved efficiency and expanded scope. J Org Chem 2005, 70, 2835–2838. [Google Scholar]
- Adam, D. Out of the kitchen. Nature 2003, 421, 571–572. [Google Scholar]
- Caddick, S. Microwave assisted organic reactions. Tetrahedron 1995, 51, 10403–10432. [Google Scholar]
- Mazzocchia, C; Modica, G; Kaddouri, A; Nannicini, R. Fatty acid methyl esters synthesis from triglycerides over heterogeneous catalysts in the presence of microwaves. Comptes Rendus Chimie 2004, 7, 601–605. [Google Scholar]
- Shieh, W-C; Dell, S; Repic, O. Large scale microwave-accelerated esterification of carboxylic acids with dimethyl carbonate. Tetrahedron Lett 2002, 43, 5607–5609. [Google Scholar]
- Freemantle, M. BASF's smart ionic liquid. C&EN 2003, 81, 9. [Google Scholar]
- Weyershausen, B; Hell, K; Hesse, U. Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities (Transformations and Processes); Rogers, RD, Seddon, KR, Eds.; American Chemical Society: Washington, DC, 2005; pp. 133–143. [Google Scholar]
- Schaefgen, JR; Newman, MS; Verhoek, FH. Ionization constants of butylamine, piperidine and triethylamine in methanol. J Am Chem Soc 1944, 66, 1847–1849. [Google Scholar]
- Lide, DR. CRC Handbook of Chemistry and Physics, 84th ed; CRC Press Inc: New York, 2003; pp. 6–3. [Google Scholar]
- Dahlen, A; Hilmersson, G. Mechanistic study of the SmI2/H2O/amine-mediated reduction of alkyl halides: Amine base strength (pKBH+) dependent rate. J Am Chem Soc 2005, 127, 8340–8347. [Google Scholar]
- Morishima, I; Fujii, H; Shiro, Y; Sano, S. Studies on the iron(II) meso-oxyporphyrin π- neutral radical as a reaction intermediate in heme catabolism. Inorg Chem 1995, 34, 1528–1535. [Google Scholar]
- Underwood, GR; Dietze, PE. Nucleophilic substitution at centers other than carbon: reaction at the chlorine of N-chloroacetanilides with triethylamine as the nucleophile. J Org Chem 1984, 49, 5225–5229. [Google Scholar]
- Kobayashi, S; Tsutsui, M; Mukaiyama, T. A convenient method for the transformation of alcohols to alkyl iodides using 2-fluoropyridinium salt. Chem Lett 1976, 373–374. [Google Scholar]
- Mukaiyama, T; Hojo, K. Optical interconversion of enantiomeric secondary alcohols using 2- fluorobenzothiazolium salt. Chem Lett 1976, 893–896. [Google Scholar]
- Huang, HT; Foster, RJ; Niemann, C. The kinetics of the α-chymotrypsin-catalyzed hydrolysis of acetyl- and nicotinyl-L-phenylalaninamide in aqueous solutions at 25° and pH 7.91. J Am Chem Soc 1952, 74, 105–109. [Google Scholar]
- Boaz, NW; Mackenzie, EB; Debenham, SD; Large, SE; Ponasik, JA. Synthesis and application of phosphinoferrocenylaminophosphine ligands for asymmetric catalysis. J Org Chem 2005, 70, 1872–1880. [Google Scholar]
- Ebbersa, EJ; Ariaansa, GJA; Houbiersa, JPM; Bruggink, A; Zwanenburg, B. Controlled racemization of optically active organic compounds: Prospects for asymmetric transformation. Tetrahedron 1997, 53, 9417–9476. [Google Scholar]

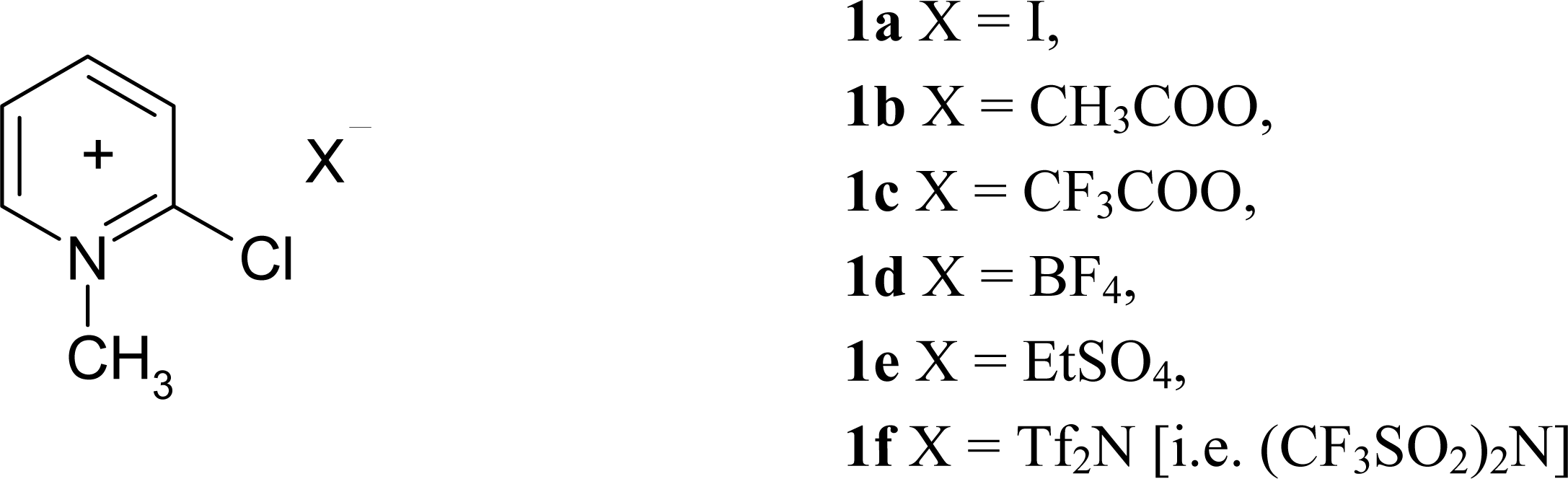

| Entry | Mukaiyama's reagent (2.4 mmol) | Solvent (5.0 mL) | Alcohol (excess) | Base (4.8 mmol) | Yield (%) 3 |
|---|---|---|---|---|---|
| 1 | [2-ClMePy]I 2 | MeOH | MeOH | TBA 4 | 14 |
| 2 | [2-ClMePy]I | MeOH | MeOH | TEA 5 | 32 |
| 3 | [2-ClMePy]I | MeOH | MeOH | MIM 6 | 77 |
| 4 | [2-ClMePy]I | DCM 7 | MeOH | MIM | 12 |
| (4.0 mmol) | |||||
| 5 | [2-ClMePy]I | EtOH | EtOH | MIM | 37 |
| 6 | [2-ClMePy]I | MeOH | MeOH | MIM | 25 |
| (3.6 mmol) |
| Entry | Mukaiyama's reagent | m.p. (°C) | Yield (%)2 |
|---|---|---|---|
| 3 | [2-ClMePy]I | 200 (dec.) | 77 |
| 7 | [2-ClMePy][EtSO4] | Liquid | 52 |
| 8 | [2-ClMePy][BF4] | 70–75 | 38 |
| 9 | [2-ClMePy][CF3COO] | Liquid | 37 |
| 10 | [2-ClMePy][CH3COO] | Liquid | 37 |
| 11 | [2-ClMePy][Tf2N] | 74–76 | 53 |
| 12 | [2-BrEtPy][BF4] | 102–104 | 60 |
| 13 | [2-FMePy][OTs] | 130–134 | 30 |
Share and Cite
Zhao, H.; Song, Z.; Cowins, J.V.; Olubajo, O. Microwave-Assisted Esterification of N-Acetyl-L-Phenylalanine Using Modified Mukaiyama’s Reagents: A New Approach Involving Ionic Liquids. Int. J. Mol. Sci. 2008, 9, 33-44. https://doi.org/10.3390/ijms9010033
Zhao H, Song Z, Cowins JV, Olubajo O. Microwave-Assisted Esterification of N-Acetyl-L-Phenylalanine Using Modified Mukaiyama’s Reagents: A New Approach Involving Ionic Liquids. International Journal of Molecular Sciences. 2008; 9(1):33-44. https://doi.org/10.3390/ijms9010033
Chicago/Turabian StyleZhao, Hua, Zhiyan Song, Janet V. Cowins, and Olarongbe Olubajo. 2008. "Microwave-Assisted Esterification of N-Acetyl-L-Phenylalanine Using Modified Mukaiyama’s Reagents: A New Approach Involving Ionic Liquids" International Journal of Molecular Sciences 9, no. 1: 33-44. https://doi.org/10.3390/ijms9010033




