Developments in Molecular Recognition and Sensing at Interfaces
Abstract
:1. Introduction
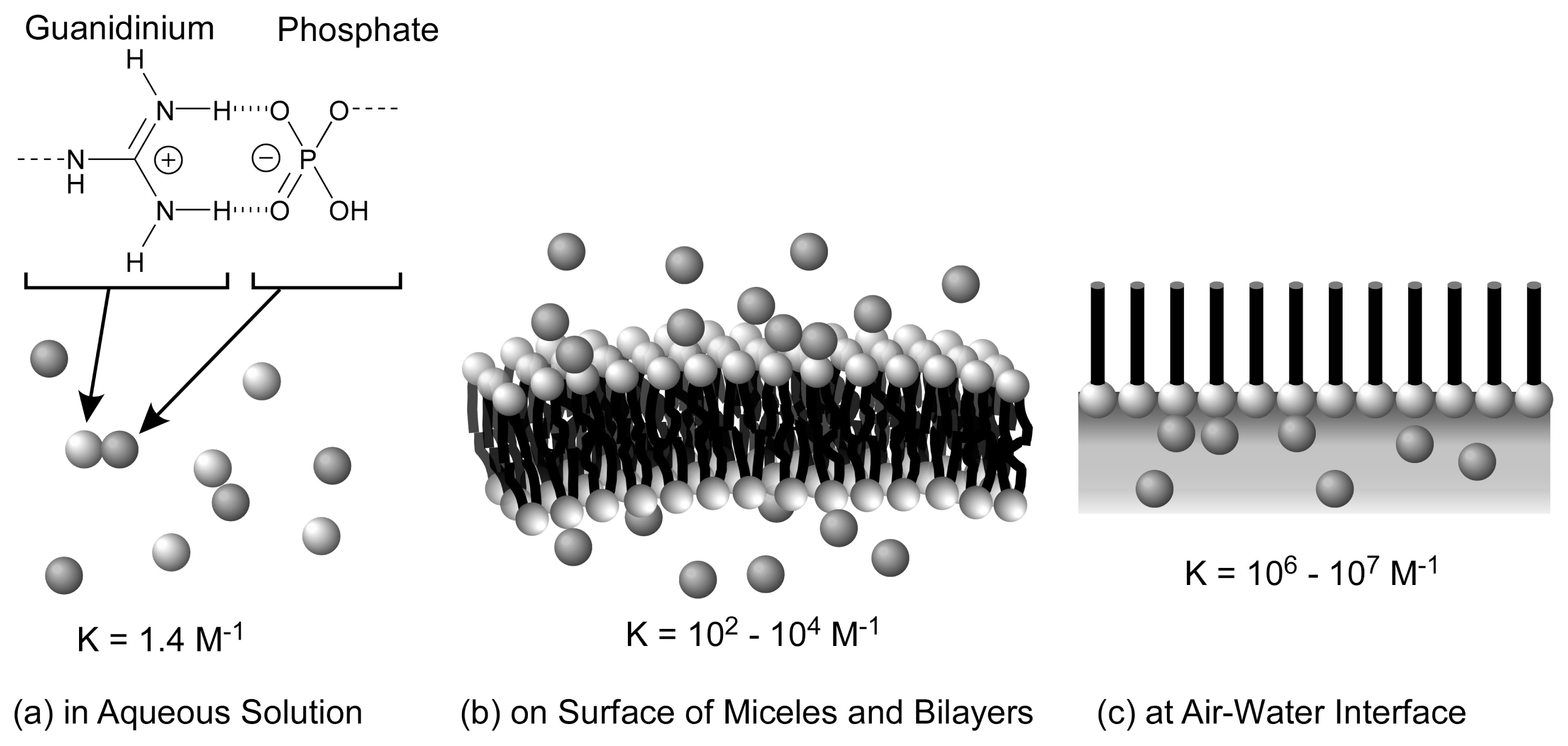
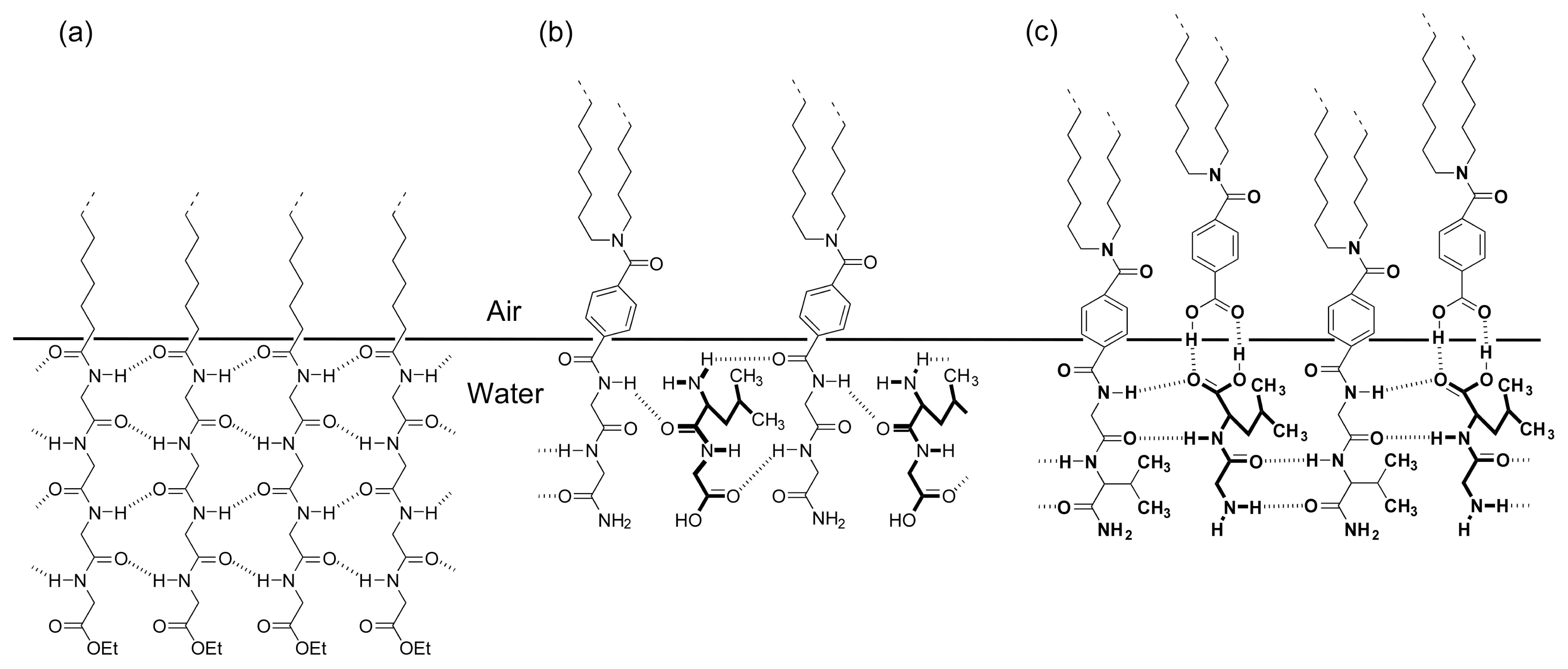
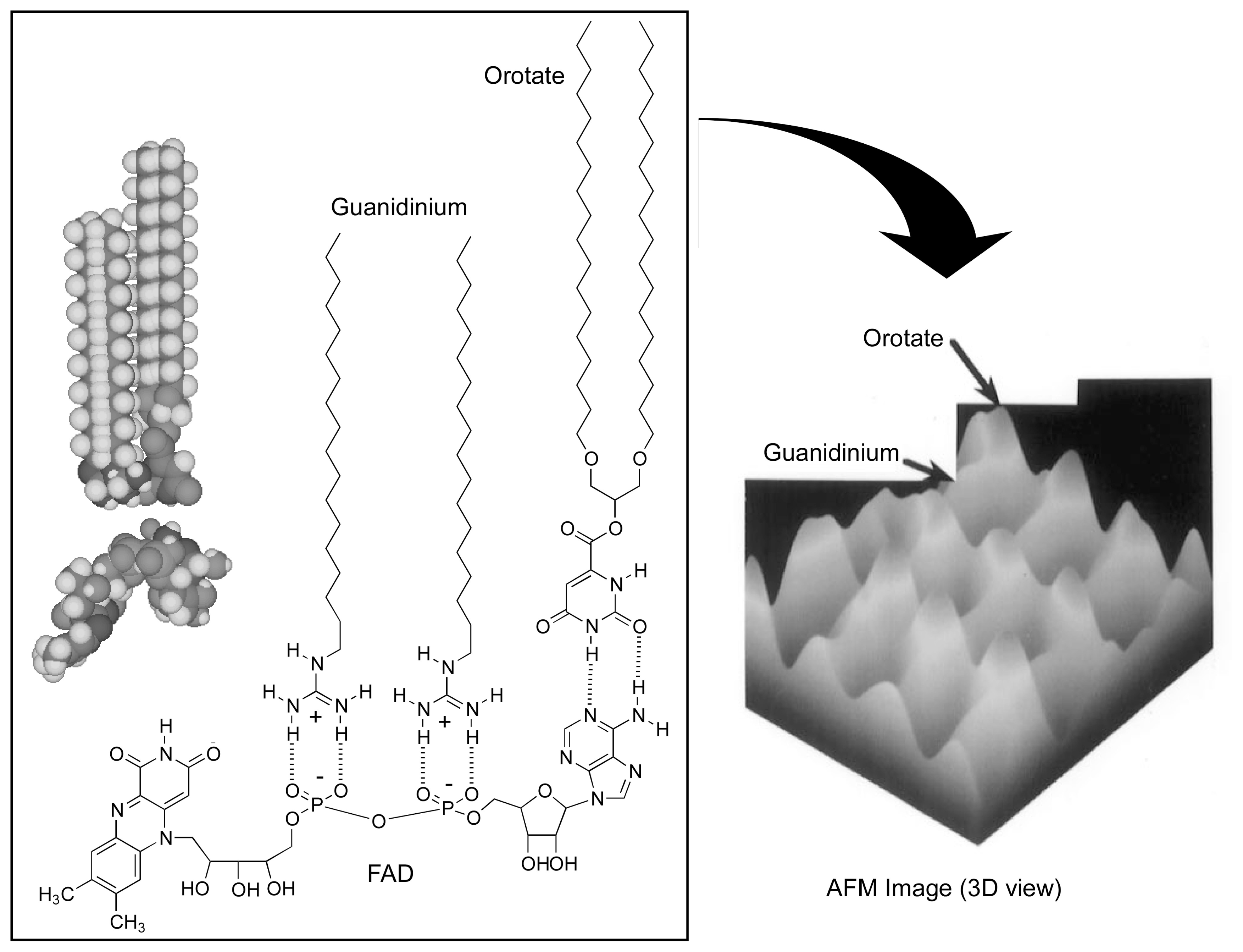
2. Molecular Recognition at the Air-Water Interface and Related Interfaces
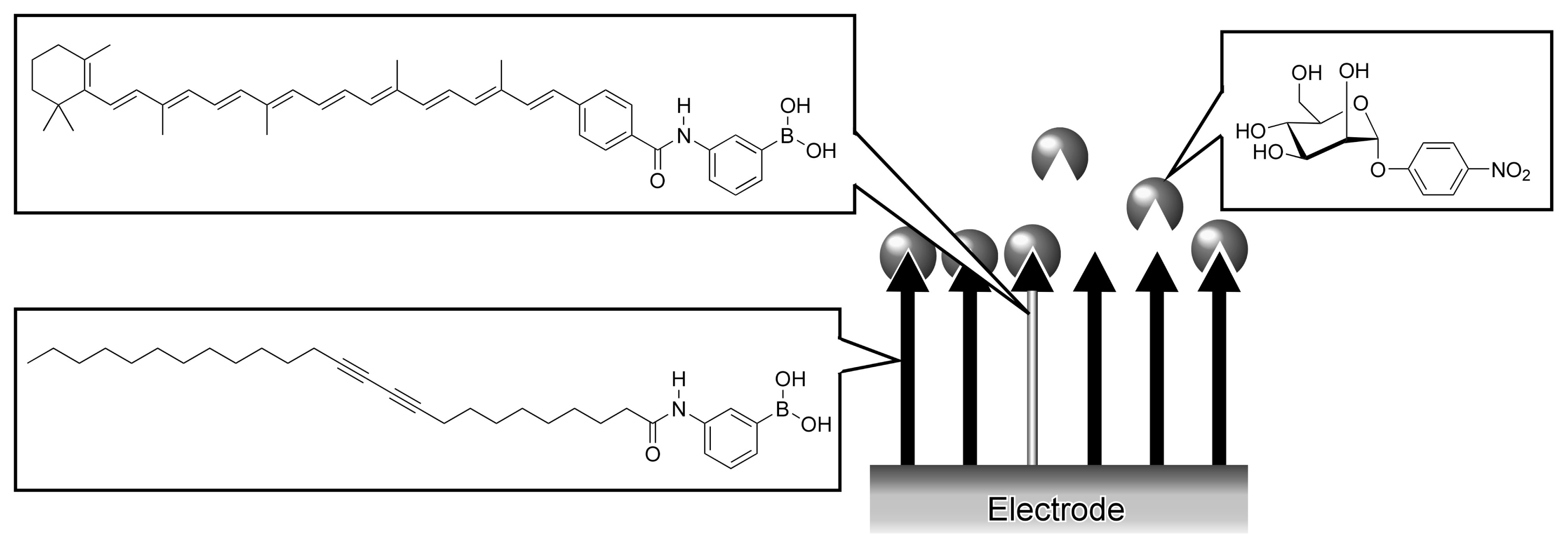
3. Molecular Recognition at Other Interfaces
4. Future Perspectives
Acknowledgements
References and Notes
- Ariga, K.; Anslyn, E.V. Manipulating the stoichiometry and strength of phosphodiester binding to a bisguanidine cleft in DMSO/water solutions. J. Org. Chem 1992, 57, 417–419. [Google Scholar]
- Kneeland, D. M.; Ariga, K.; Lynch, V.M.; Huang, C.Y.; Anslyn, E.V. Bis(alkylguanidinium) receptors for phosphodiesters: effect of counterions, solvent mixtures, and cavity flexibility on complexation. J. Am. Chem. Soc 1993, 115, 10042–10055. [Google Scholar]
- Smith, J.; Ariga, K.; Anslyn, E.V. Enhanced imidazole-catalyzed RNA cleavage induced by a bis-alkylguanidinium receptor. J. Am. Chem. Soc 1993, 115, 362–364. [Google Scholar]
- Onda, M.; Yoshihara, K.; Koyano, H.; Ariga, K.; Kunitake, T. Molecular recognition of nucleotides by the guanidinium unit at the surface of aqueous micelles and bilayers. A comparison of microscopic and macroscopic interfaces. J. Am. Chem. Soc 1996, 118, 8524–8530. [Google Scholar]
- Springs, B.; Haake, P. Equilibrium-constants for association of guanidinium and ammonium ions with oxyanions: effect of changing basicity of oxyanion. Bioorg. Chem 1977, 6, 181–190. [Google Scholar]
- Sasaki, D.Y.; Kurihara, K.; Kunitake, T. Specific, multiple point binding of ATP and AMP to a guanidinium-functionalized monolayer. J. Am. Chem. Soc 1991, 113, 9685–9686. [Google Scholar]
- Sakurai, M.; Tamagawa, H.; Furuki, T.; Inoue, Y.; Ariga, K.; Kunitake, T. A theoretical interpretation of remarkable enhancement of intermolecular binding at the lipid-water interface. Chem. Lett. 1995, 1001–1002. [Google Scholar]
- Sakurai, M.; Tamagawa, H.; Inoue, Y.; Ariga, K.; Kunitake, T. Theoretical study of intermolecular interaction at the lipid-water Interface. 1. Quantum chemical analysis using a reaction field theory. J. Phys. Chem. B 1997, 101, 4810–4816. [Google Scholar]
- Tamagawa, H.; Sakurai, M.; Inoue, Y.; Ariga, K.; Kunitake, T. Theoretical study of intermolecular interaction at the lipid-water interface. 2. Analysis based on the Poisson-Boltzmann equation. J. Phys, Chem. B 1997, 101, 4817–4825. [Google Scholar]
- Taneva, S.; Ariga, K.; Tagaki, W.; Okahata, Y. Association of amphiphilic cyclodextrins with dipalmitoylphosphatidylcholine in mixed insoluble monolayers at the air-water interface. J. Colloid Interface Sci 1989, 131, 561–566. [Google Scholar]
- Taneva, S.; Ariga, K.; Okahata, Y.; Tagaki, W. Association between amphiphilic cyclodextrins and cholesterol in mixed insoluble monolayers at the air-water interface. Langmuir 1989, 5, 111–113. [Google Scholar]
- Kruppa, M.; König, B. Reversible coordinative bonds in molecular recognition. Chem. Rev 2006, 106, 3520–3560. [Google Scholar]
- Ebara, Y.; Ebato, H.; Ariga, K.; Okahata, Y. Interactions of calcium ions with phospholipid membranes. Studies on π-A isotherms and electrochemical and quartz-crystal microbalance measurements. Langmuir 1994, 10, 2267–2271. [Google Scholar]
- Ariga, K.; Kunitake, T.; Furuta, H. Specific binding of iodide ion to N-confused tetraphenylporphyrin (NC-TPP) at the air-water interface. J. Chem. Soc., Perkin Trans. 2 1996, 667–672. [Google Scholar]
- Huo, Q.; Sui, G.; Zheng, Y.; Kele, P.; Leblanc, R.M.; Hasegawa, T.; Nishijo, J.; Umemura, J. Metal complexation with Langmuir monolayers of histidyl peptide lipids. Chem. Eur. J 2001, 22, 4796–4804. [Google Scholar]
- Prabhakaran, D.; Yuehong, M.; Nanjo, H.; Matsunaga, H. Naked-eye cadmium sensor: using chromoionophore arrays of Langmuir-Blodgett molecular assemblies. Anal. Chem 2007, 79, 4056–4065. [Google Scholar]
- Ariga, K.; Shin, J.S.; Kunitake, T. Interaction of lipid monolayers with aqueous neutral polymers and the consequent monolayer stabilization and improved Langmuir-Blodgett transfer. J. Colloid Interface Sci 1995, 170, 440–448. [Google Scholar]
- Qian, P.; Matsuda, M.; Miyashita, T. Chiral molecular recognition in polymer Langmuir-Blodgett films containing axially chiral binaphthyl groups. J. Am. Chem. Soc 1993, 115, 5624–5628. [Google Scholar]
- Kitano, H.; Ringsdorf, H. Surface behaviors of nucleic acid base-containing lipids in monolayer and bilayer systems. Bull. Chem. Soc. Jpn 1985, 58, 2826–2828. [Google Scholar]
- Ariga, K.; Kunitake, T. Molecular recognition at air-water and related interfaces: complementary hydrogen bonding and multisite interaction. Acc. Chem. Res 1998, 31, 371–378. [Google Scholar]
- Ariga, K.; Kamino, A.; Koyano, H.; Kunitake, T. Recognition of aqueous flavin mononucleotide on the surface of binary monolayers of guanidinium and melamine amphiphiles. J. Mater. Chem 1997, 7, 1155–1161. [Google Scholar]
- Taguchi, K.; Ariga, K.; Kunitake, T. Multi-site recognition of flavin adenine dinucleotide by mixed monolayers on water. Chem. Lett. 1995, 701–702. [Google Scholar]
- Vollhardt, D.; Liu, F.; Rudert, R.; He, W. Interfacial molecular recognition of dissolved thymine by medium chain dialkyl melamine-type monolayers. J. Phys. Chem. B 2005, 109, 10849–10857. [Google Scholar]
- Vollhardt, D.; Liu, F.; Rudert, R. Molecular recognition of dissolved pyrimidine derivatives by a dialkyl melamine-type monolayer. ChemPhysChem 2005, 6, 1246–1250. [Google Scholar]
- Miao, W.; Du, X.; Liang, Y. Molecular recognition of 1-(2-octadecyloxycarbonylethyl)cytosine monolayers to guanosine at the air-water interface investigated by infrared reflection-absorption spectroscopy. J. Phys. Chem. B 2003, 107, 13636–13642. [Google Scholar]
- Wang, Y.; Du, X.; Miao, W.; Liang, Y. Molecular recognition of cytosine- and guanine-functionalized nucleolipids in the mixed monolayers at the air-water interface and Langmuir-Blodgett films. J. Phys. Chem. B 2006, 110, 4914–4923. [Google Scholar]
- Cha, X.; Ariga, K.; Kunitake, T. Inter-peptide hydrogen bonding in monolayers of oligoglycine amphiphiles. Bull. Chem. Soc. Jpn 1996, 69, 163–168. [Google Scholar]
- Cha, X.; Ariga, K.; Onda, M.; Kunitake, T. Molecular recognition of aqueous dipeptides by noncovalently aligned oligoglycine units at the air/water interface. J. Am. Chem. Soc 1995, 117, 11833–11838. [Google Scholar]
- Cha, X.; Ariga, K.; Kunitake, T. Multi-site binding of aqueous dipeptides by mixed monolayers at the air-water interface. Chem. Lett. 1996, 73–74. [Google Scholar]
- Cha, X.; Ariga, K.; Kunitake, T. Molecular recognition of aqueous dipeptides at multiple hydrogen-bonding sites of mixed peptide monolayers. J. Am. Chem. Soc 1996, 118, 9545–9551. [Google Scholar]
- Ariga, K.; Kamino, A.; Cha, X.; Kunitake, T. Multisite recognition of aqueous dipeptides by oligoglycine arrays mixed with guanidinium and other receptor units at the air-water interface. Langmuir 1999, 15, 3875–3885. [Google Scholar]
- Wang, C.; Li, C.; Ji, X.; Orbulescu, J.; Xu, J.; Leblanc, R.M. Peptidolipid as binding site of acetylcholinesterase: molecular recognition of paraoxon in Langmuir films. Langmuir 2006, 22, 2200–2204. [Google Scholar]
- Wang, C.; Zheng, J.; Oliveira, O.N., Jr.; Leblanc, R.M. Nature of the interaction between a peptidolipid Langmuir monolayer and paraoxon in the subphase. J. Phys. Chem. C 2007, 111, 7826–7833. [Google Scholar]
- Izhaky, D; Addadi, L. Stereoselective interactions of a specialized antibody with cholesterol and epicholesterol monolayers. Chem. Eur. J 2000, 6, 869–874. [Google Scholar]
- Zadmard, R.; Schrader, T. Nanomolar protein sensing with embedded receptor molecules. J. Am. Chem. Soc 2005, 127, 904–915. [Google Scholar]
- Schuster, B.; Gufler, P.C.; Pum, D.; Sleytr, U.B. Interplay of phospholipase A2 with S-layer-supported lipid monolayers. Langmuir 2003, 19, 3393–3397. [Google Scholar]
- Pavinatto, F.J.; Caseli, L.; Pavinatto, A.; dos Santos, D.S., Jr.; Nobre, T.M.; Zaniquelli, M.E.D.; Silva, H.S.; Miranda, P.B.; de Oliveira, O.N., Jr. Probing chitosan and phospholipid interactions using Langmuir and Langmuir-Blodgett films as cell membrane models. Langmuir 2007, 23, 7666–7671. [Google Scholar]
- Matsuura, K.; Kitakouji, H.; Sawada, N.; Ishida, H.; Kiso, M.; Kitajima, K.; Kobayashi, K. A quantitative estimation of carbohydrate-carbohydrate interaction using clustered oligosaccharides of glycolipid monolayers and of artificial glycoconjugate polymers by surface plasmon resonance. J. Am. Chem. Soc 2000, 122, 7406–7407. [Google Scholar]
- Kurihara, K.; Ohto, K.; Tanaka, Y.; Aoyama, Y.; Kunitake, T. Molecular recognition of sugars by monolayers of resorcinol-dodecanal cyclotetramer. J. Am. Chem. Soc 1991, 113, 444–450. [Google Scholar]
- Ludwig, R.; Ariga, K.; Shinkai, S. Sensitive detection of saccharides by an amphiphilic phenylboronic acid at the air-water interface in the presence of quaternized amines. Chem. Lett. 1993, 1413–1416. [Google Scholar]
- Ariga, K.; Isoyama, K.; Hayashida, O.; Aoyama, Y.; Okahata, Y. A QCM study on adsorption of macrocyclic sugar-cluster to variously-functionalized monolayers. Chem. Lett. 1998, 1007–1008. [Google Scholar]
- Miyahara, T.; Kurihara, K. Electroconductive Langmuir-Blodgett films containing a carotenoid amphiphile for sugar recognition. J. Am. Chem. Soc 2004, 126, 5684–5685. [Google Scholar]
- Ariga, K. Template-assisted nano-patterning from submicron-scale to submolecular-level. J. Nanosci. Nanotechnol 2004, 4, 23–34. [Google Scholar]
- Ariga, K.; Nakanishi, T.; Takagi, N.; Tanaka, R.; Kikuchi, J. Two-dimensional molecular patterns and their dynamic functions: molecular recognition of aqueous guest by mixed monolayer of alkyl cyclophane and amphiphilic guanidinium. Colloid Surf. A-Physicochem. Eng. Asp 2006, 284, 499–504. [Google Scholar]
- Kamino, A.; Koyano, H.; Ariga, K.; Kunitake, T. Control of the molecular packing in guanidinium monolayers through binding with aqueous polycarboxylates. Bull. Chem. Soc. Jpn 1996, 69, 3619–3631. [Google Scholar]
- Oishi, Y.; Kato, T.; Kuramori, M.; Suehiro, K.; Ariga, K.; Kamino, A.; Koyano, H.; Kunitake, T. Control of molecular ordering in guanidinium-functionalized monolayer by carboxylate template molecules. Chem. Commun. 1997, 1357–1358. [Google Scholar]
- Marchi-Artzner, V.; Artzner, F.; Karthaus, O.; Shimomura, M.; Ariga, K.; Kunitake, T.; Lehn, J.-M. Molecular recognition between 2,4,6-triaminopyrimidine lipid monolayers and complementary barbituric molecules at the air/water interface: effects of hydrophilic spacer, ionic strength, and pH. Langmuir 1998, 14, 5164–5171. [Google Scholar]
- Koyano, H.; Bissel, P.; Yoshihara, K.; Ariga, K.; Kunitake, T. Syntheses and interfacial hydrogen-bonded network of hexaalkyl tris(melamine) amphiphiles. Langmuir 1997, 13, 5426–5432. [Google Scholar]
- Koyano, H.; Bissel, P.; Yoshihara, K.; Ariga, K.; Kunitake, T. Effect of melamine-amphiphile structure on the extent of two-dimensional hydrogen-bonded networks incorporating barbituric acid. Chem. Eur. J 1997, 3, 1077–1082. [Google Scholar]
- Kovalchuk, N.M.; Vollhardt, D.; Fainerman, V.B.; Aksenenko, E.V. Recognition and dissociation kinetics in the interfacial molecular recognition of barbituric acid by amphiphilic melamine-type monolayers. J. Phys. Chem. B 2007, 111, 8283–8289. [Google Scholar]
- Koyano, H.; Yoshihara, K.; Ariga, K.; Kunitake, T.; Oishi, Y.; Kawano, O.; Kuramori, M.; Suehiro, K. Atomic force microscopic observation of a dialkylmelamine monolayer on barbituric acid. Chem. Commun. 1996, 1769–1770. [Google Scholar]
- Oishi, Y.; Torii, Y.; Kuramori, M.; Suehiro, K.; Ariga, K.; Taguchi, K.; Kamino, A.; Kunitake, T. Two-dimensional molecular patterning through molecular recognition. Chem. Lett. 1996, 411–412. [Google Scholar]
- Oishi, Y.; Torii, Y.; Kato, T.; Kuramori, M.; Suehiro, K.; Ariga, K.; Taguchi, K.; Kamino, A.; Koyano, H.; Kunitake, T. Molecular patterning of a guanidinium/orotate mixed monolayer through molecular recognition with flavin adenine dinucleotide. Langmuir 1997, 13, 519–524. [Google Scholar]
- Ariga, K.; Nakanishi, T.; Hill, J.P. A paradigm shift in the field of molecular recognition at the air-water interface: from static to dynamic. Soft Matter 2006, 2, 465–477. [Google Scholar]
- Higuchi, M.; Wright, J.P.; Taguchi, K.; Kinoshita, T. Structure and molecular recognition properties of a poly(allylamine) monolayer containing poly(L-alanine) graft chains. Langmuir 2000, 16, 7061–7065. [Google Scholar]
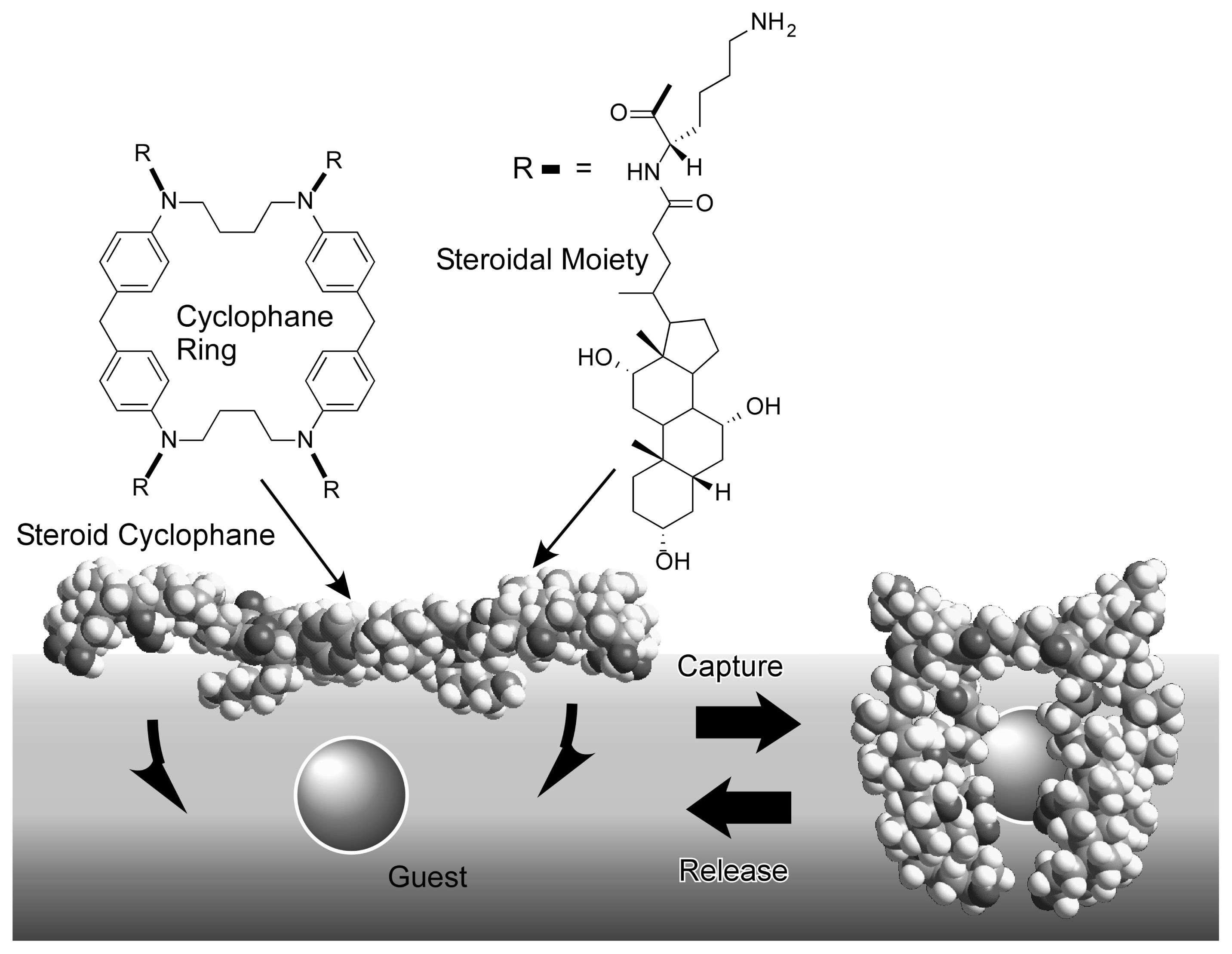
- Ariga, K.; Terasaka, Y.; Sakai, D.; Tsuji, H.; Kikuchi, J. Piezoluminescence based on molecular recognition by dynamic cavity array of steroid cyclophanes at the air-water interface. J. Am. Chem. Soc 2000, 122, 7835–7836. [Google Scholar]
- Ariga, K.; Tanaka, R.; Takagi, N.; Kikuchi, J. Molecular recognition by cyclophane/guanidinium supramolecular receptor embedded at the air-water interface. Supramol. Chem 2003, 15, 87–94. [Google Scholar]
- Ariga, K.; Nakanishi, T.; Terasaka, Y.; Tsuji, H.; Sakai, D.; Kukuchi, J. Piezoluminescence at the air-water interface through dynamic molecular recognition driven by lateral pressure application. Langmuir 2005, 21, 976–981. [Google Scholar]
- Ariga, K.; Nakanishi, T.; Hill, J.P.; Terasaka, Y.; Sakai, D.; Kikuchi, J. Effect of guest capture modes on molecular recognition by a dynamic cavity array at the air-water interface: soft vs. tight and fast vs. slow. Soft Matter 2005, 1, 132–137. [Google Scholar]
- Ariga, K.; Nakanishi, T.; Terasaka, Y.; Kikuchi, J. Catching a molecule at the air-water interface: dynamic pore array for molecular recognition. J. Porous Mater 2006, 13, 427–430. [Google Scholar]
- Ariga, K.; Sakai, D.; Terasaka, Y.; Tsuji, H.; Kikuchi, J. Information conversion on molecular assemblies containing steroid cyclophanes. Thin Solid Films 2001, 393, 291–297. [Google Scholar]
- Azov, V.A.; Beeby, A.; Cacciarini, M.; Cheetham, A.G.; Diederich, F.; Frei, M.; Gimzewski, J.K.; Gramlich, V.; Hecht, B.; Jaun, B.; Latychevskaia, T.; Lieb, A.; Lill, Y.; Marotti, F.; Schlegel, A.; Schlittler, R.R.; Skinner, P.J.; Seiler, P.; Yamakoshi, Y. Resorcin[4]arene cavitand-based molecular switches. Adv. Funct. Mater 2006, 16, 147–156. [Google Scholar]
- Huang, T.J.; Tseng, H.R.; Sha, L.; Lu, W.; Brough, B.; Flood, A.H.; Yu, B.-D.; Celestre, P.C.; Chang, J.P.; Stoddart, J.F.; Ho, C.M. Mechanical shuttling of linear motor-molecules in condensed phases on solid substrates. Nano Lett 2004, 4, 2065–2071. [Google Scholar]
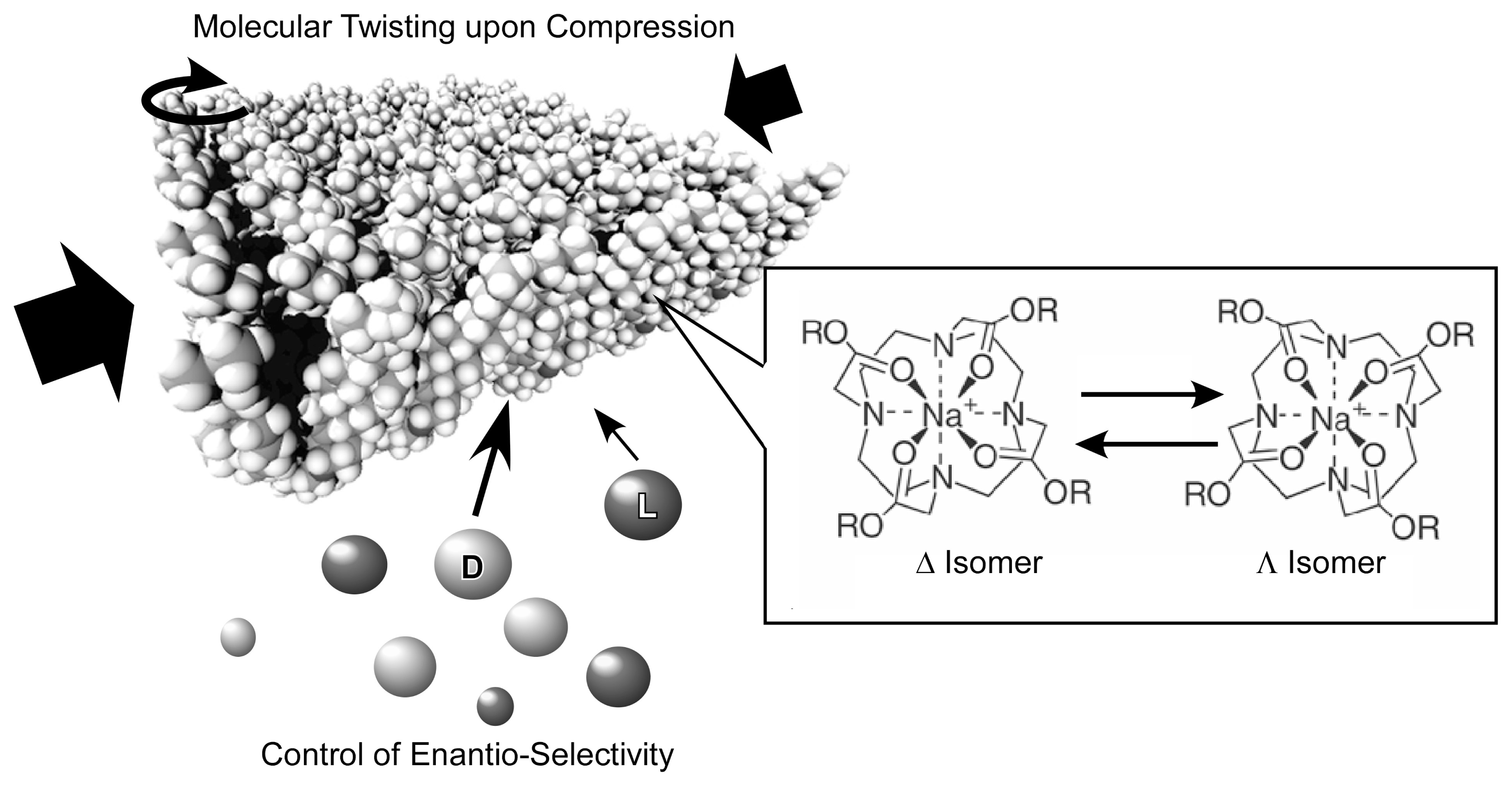
- Michinobu, T.; Shinoda, S.; Nakanishi, T.; Hill, J.P.; Fujii, K.; Player, T.N.; Tsukube, H.; Ariga, K. Mechanical control of enantioselectivity of amino acid recognition by cholesterol-armed cyclen monolayer at the air-water interface. J. Am. Chem. Soc 2006, 128, 14478–14479. [Google Scholar]
- Flink, S.; van Veggel, F.C.J.M.; Reinhoudt, D.N. Sensor functionalities in self-assembled monolayers. Adv. Mater 2000, 12, 1315–1328. [Google Scholar]
- Okahata, Y.; Ariga, K.; Nakahara, H.; Fukuda, K. Permeation control by a phase transition of the dialkylsilane monolayer immobilized on a porous glass plate. J. Chem. Soc., Chem. Commun. 1986, 1069–1071. [Google Scholar]
- Okahata, Y.; Ariga, K.; Shimizu, O. Porous glass plate immobilized with the adsorbed monolayer of dialkylsilane amphiphiles. Permeation control by a phase transition of the adsorbed monolayer. Langmuir 1986, 2, 538–540. [Google Scholar]
- Ariga, K.; Okahata, Y. Polymerized monolayers of single-, double-, and triple-chain silane amphiphiles and permeation control through the monolayer-immobilized porous glass plate in an aqueous solution. J. Am. Chem. Soc 1989, 111, 5618–5622. [Google Scholar]
- Okahata, Y.; Yokobori, M.; Ebara, Y.; Ebato, H.; Ariga, K. Electrochemical properties of covalently bonded silane amphiphile monolayers on a tin dioxide electrode. Langmuir 1990, 6, 1148–1153. [Google Scholar]
- Gibbs-Davis, J.M.; Hayes, P.L.; Scheidt, K.A.; Geiger, F. M. Anion chelation by amido acid functionalized fused quartz/water interfaces studied by nonlinear optics. J. Am. Chem. Soc 2007, 129, 7175–7184. [Google Scholar]
- Credo, G.M.; Boal, A.K.; Das, K.; Galow, T.H.; Rotello, V.M.; Feldheim, D.L.; Gorman, C.B. Supramolecular assembly on surfaces: manipulating conductance in noncovalently modified mesoscale structures. J. Am. Chem. Soc 2002, 124, 9036–9037. [Google Scholar]
- Kitano, H.; Taira, Y. Inclusion of bisphenols by a self-assembled monolayer of thiolated cyclodextrin on a gold electrode. Langmuir 2002, 18, 5835–5840. [Google Scholar]
- Endo, H.; Nakaji-Hirabayashi, T.; Morokoshi, S.; Gemmei-Ide, M.; Kitano, H. Orientational effect of surface-confined cyclodextrin on the inclusion of bisphenols. Langmuir 2005, 21, 1314–1321. [Google Scholar]
- Nakaji-Hirabayashi, T.; Endo, H.; Kawasaki, H.; Gemmei-Ide, M.; Kitano, H. Inclusion of bisphenols by a self-assembled monolayer of thiolated calix[6]arene on a gold surface. Environ. Sci. Technol 2005, 39, 5414–5420. [Google Scholar]
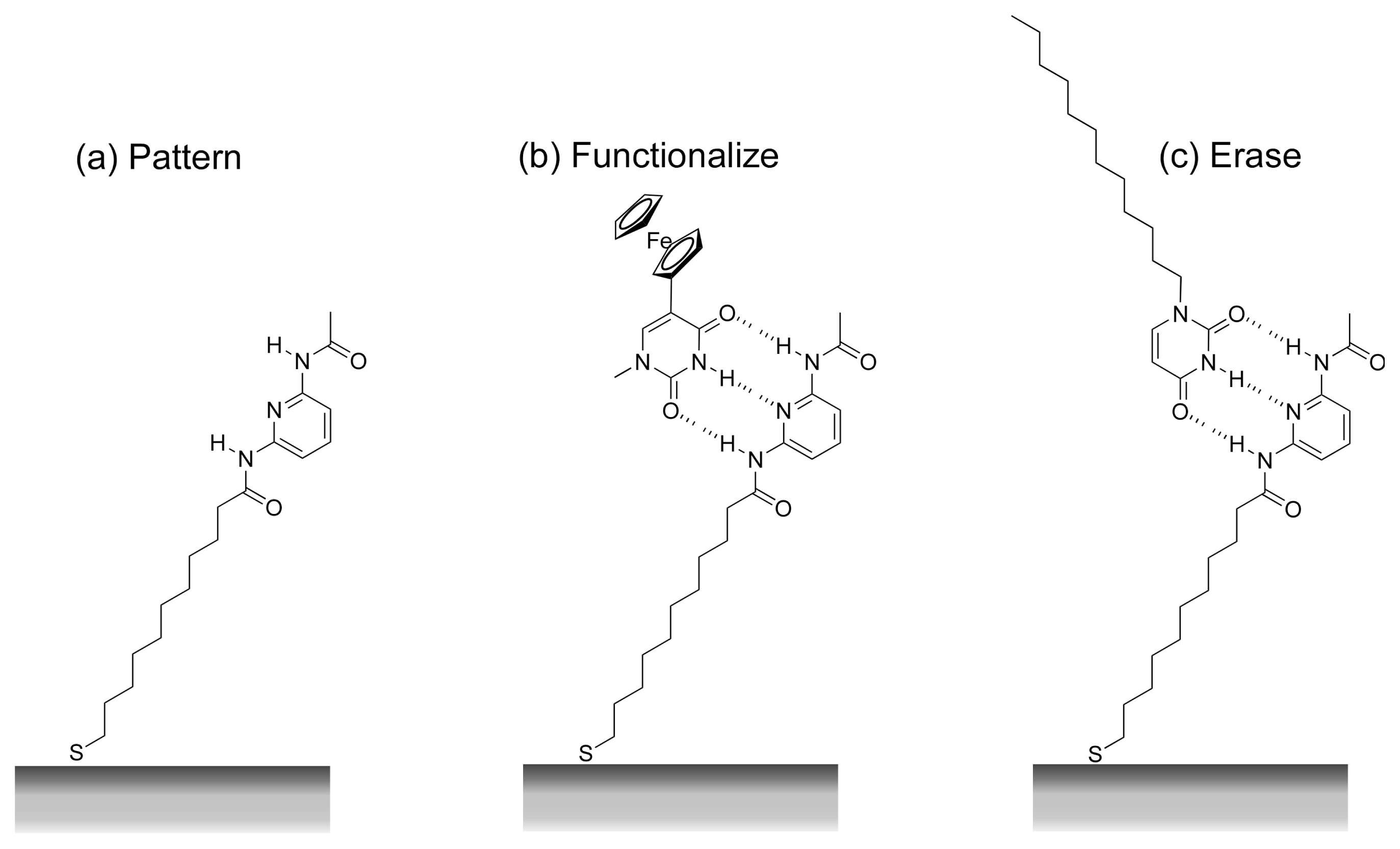
- Lahav, M.; Katz, E.; Willner, I. Photochemical imprint of molecular recognition sites in two-dimensional monolayers assembled on Au electrodes: effects of the monolayer structures on the binding affinities and association kinetics to the imprinted interfaces. Langmuir 2001, 17, 7387–7395. [Google Scholar]
- Shen, Z.; Huang, M.; Xiao, C.; Zhang, Y.; Zeng, X.; Wang, P.G. Nonlabeled quartz crystal microbalance biosensor for bacterial detection using carbohydrate and lectin recognitions. Anal. Chem 2007, 79, 2312–2319. [Google Scholar]
- Metallo, S. J.; Kane, R.S.; Holmlin, R.E.; Whitesides, G.M. Using bifunctional polymers presenting Vancomycin and fluorescein groups to direct anti-fluorescein antibodies to self-assembled monolayers presenting D-alanine-D-alanine groups. J. Am. Chem. Soc 2003, 125, 4534–4540. [Google Scholar]
- Liu, Z.; Amiridis, M.D. Quantitative FT-IRRAS spectroscopic studies of the interaction of avidin with biotin on functionalized quartz surfaces. J. Phys. Chem. B 2005, 109, 16866–16872. [Google Scholar]
- Taranekar, P.; Baba, A.; Park, J.Y.; Fulghum, T.M.; Advincula, R. Dendrimer precursors for nanomolar and picomolar real-time surface plasmon resonance/potentiometric chemical nerve agent sensing using electrochemically crosslinked ultrathin films. Adv. Funct. Mater 2006, 16, 2000–2007. [Google Scholar]
- Bunker, B.C.; Huber, D.L.; Kushmerick, J.G.; Dunbar, T.; Kelly, M.; Matzke, C.; Cao, J.; Jeppesen, J.O.; Perkins, J.; Flood, A.H.; Stoddart, J.F. Switching surface chemistry with supramolecular machines. Langmuir 2007, 23, 31–34. [Google Scholar]
- You, C.-C.; Verma, A.; Rotello, V.M. Engineering the nanoparticle–biomacromolecule interface. Soft Matter 2006, 2, 190–204. [Google Scholar]
- Guerrini, L.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Functionalization of Ag nanoparticles with dithiocarbamate calix[4]arene As an effective supramolecular host for the surface-enhanced Raman scattering detection of polycyclic aromatic hydrocarbons. Langmuir 2006, 22, 10924–10926. [Google Scholar]
- Ishizaka, S.; Kinoshita, S.; Nishijima, Y.; Kitamura, N. Direct observation of molecular recognition mediated by triple hydrogen bonds at a water/oil interface: time-resolved total internal reflection fluorometry study. Anal. Chem 2003, 75, 6035–6042. [Google Scholar]
- Ohashi, A.; Tsukahara, S.; Watarai, H. Molecular recognition of diazine isomers and purine bases by the aggregation of palladium(II)-pyridylazo complex at the toluene/water interface. Langmuir 2003, 19, 4645–4651. [Google Scholar]
- Nochi, K.; Yamaguchi, A.; Hayashita, T.; Uchida, T.; Teramae, N. Direct observation of alkali metal ion recognition processes at the heptane/water interface by second harmonic generation spectroscopy. J. Phys. Chem. B 2002, 106, 9906–9911. [Google Scholar]
- Paleos, C.M.; Tsiourvas, D. Molecular recognition of organized assemblies via hydrogen bonding in aqueous media. Adv. Mater 1997, 9, 695–710. [Google Scholar]
- Kikuchi, J.; Ariga, K.; Miyazaki, T.; Ikeda, K. An artificial signal transduction system. Control of lactate dehydrogenase activity performed by an artificial cell-surface receptor. Chem. Lett. 1999, 253–254. [Google Scholar]
- Kikuchi, J.; Ariga, K.; Ikeda, K. Signal transduction mediated by artificial cell-surface receptors: activation of lactate dehydrogenase triggered by molecular recognition and phase reorganization of bile acid derivatives embedded in a synthetic bilayer membrane. Chem. Commun. 1999, 547–548. [Google Scholar]
- Kikuchi, J.; Ariga, K.; Sasaki, Y.; Ikeda, K. Control of enzymic activity by artificial cell-surface receptors. J. Mol. Catal. B-Enzym 2001, 11, 977–984. [Google Scholar]
- Fukuda, K.; Sasaki, Y.; Ariga, K.; Kikuchi, J. Dynamic behavior of a transmembrane molecular switch as an artificial cell-surface receptor. J. Mol. Catal. B-Enzym 2001, 11, 971–976. [Google Scholar]
- Pantos, A.; Tsiourvas, D.; Nounesis, G.; Paleos, C.M. Interaction of functional dendrimers with multilamellar liposomes: design of a model system for studying drug delivery. Langmuir 2005, 21, 7483–7490. [Google Scholar]
- Sasaki, D.Y.; Waggoner, T.A.; Last, J.A.; Alam, T.M. Crown ether functionalized lipid membranes: lead ion recognition and molecular reorganization. Langmuir 2002, 18, 3714–3721. [Google Scholar]
- Falvey, P.; Lim, C.W.; Darcy, R.; Revermann, T.; Karst, U.; Giesbers, M.; Marcelis, A.T.M.; Lazar, A.; Coleman, A.W.; Reinhoudt, D.N.; Jan Ravoo, B.J. Bilayer vesicles of amphiphilic cyclodextrins: host membranes that recognize guest molecules. Chem. Eur. J 2005, 11, 1171–1180. [Google Scholar]
- Kolusheva, S.; Kafri, R.; Katz, M.; Jelinek, R. Rapid colorimetric detection of antibody-epitope recognition at a biomimetic membrane interface. J. Am. Chem. Soc 2001, 123, 417–422. [Google Scholar]
- Bohrer, F.I.; Sharoni, A.; Colesniuc, C.; Park, J.; Schuller, I.K.; Kummel, A.C.; Trogler, W.C. Gas sensing mechanism in chemiresistive cobalt and metal-free phthalocyanine thin films. J. Am. Chem. Soc 2007, 129, 5640–5646. [Google Scholar]
- Han, M.; Kane, R.; Goto, M.; Belfort, G. Discriminate surface molecular recognition sites on a microporous substrate: a new approach. Macromolecules 2003, 36, 4472–4477. [Google Scholar]
- Kim, H.; Cohen, R.E.; Hammond, P.T.; Irvine, D.J. Live lymphocyte arrays for biosensing. Adv. Funct. Mater 2006, 16, 1313–1323. [Google Scholar]
- Wang, C.; Ma, Z.; Wang, T.; Su, Z. Synthesis, assembly, and biofunctionalization of silica-coated gold nanorods for colorimetric Biosensing. Adv. Funct. Mater 2006, 16, 1673–1678. [Google Scholar]
- Lee, J.; Hernandez, P.; Lee, J.; Govorov, A.O.; Kotov, N.A. Exciton–plasmon interactions inmolecular spring assemblies of nanowires and wavelength-based protein detection. Nature Mater 2007, 6, 291–295. [Google Scholar]
- Ariga, K.; Nakanishi, T.; Hill, J.P.; Shirai, M.; Okuno, M.; Abe, T.; Kikuchi, J. Tunable pK of amino acid residues at the air-water interface gives an L-zyme (Langmuir enzyme). J. Am. Chem. Soc 2005, 127, 12074–12080. [Google Scholar]
- Nakanishi, T.; Miyashita, N.; Michinobu, T.; Wakayama, Y.; Tsuruoka, T.; Ariga, K.; Kurth, D. G. Perfectly straight nanowires of fullerenes bearing long alkyl-chains on graphite. J. Am. Chem. Soc 2006, 128, 6328–6329. [Google Scholar]
- Hill, J.P.; Wakayama, Y.; Schmitt, W.; Tsuruoka, T.; Nakanishi, T.; Zandler, M.L.; McCarty, A.L.; D’Souza, F.; Milgrom, L.R.; Ariga, K. Regulating the stability of 2D crystal structures using an oxidation state-dependent molecular conformation. Chem. Commun 2006, 2320–2322. [Google Scholar]
- Hill, J.P.; Wakayama, Y.; Ariga, K. How molecules accommodate a 2D crystal lattice mismatch: an unusual ‘mixed’ conformation of tetraphenylporphyrin. Phys. Chem. Chem. Phys 2006, 8, 5034–5037. [Google Scholar]
- Vinu, A.; Hossain, K.Z.; Ariga, K. Recent advances in functionalization of mesoporous silica. J. Nanosci. Nanotechnol 2005, 5, 347–371. [Google Scholar]
- Vinu, A.; Mori, T.; Ariga, K. New families of mesoporous materials. Sci. Technol. Adv. Mater 2006, 7, 753–771. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Ariga, K.; Hill, J.P.; Endo, H. Developments in Molecular Recognition and Sensing at Interfaces. Int. J. Mol. Sci. 2007, 8, 864-883. https://doi.org/10.3390/i8080864
Ariga K, Hill JP, Endo H. Developments in Molecular Recognition and Sensing at Interfaces. International Journal of Molecular Sciences. 2007; 8(8):864-883. https://doi.org/10.3390/i8080864
Chicago/Turabian StyleAriga, Katsuhiko, Jonathan P. Hill, and Hiroshi Endo. 2007. "Developments in Molecular Recognition and Sensing at Interfaces" International Journal of Molecular Sciences 8, no. 8: 864-883. https://doi.org/10.3390/i8080864




