Solubilization of Single-walled Carbon Nanotubes with Single- stranded DNA Generated from Asymmetric PCR
Abstract
:1. Introduction
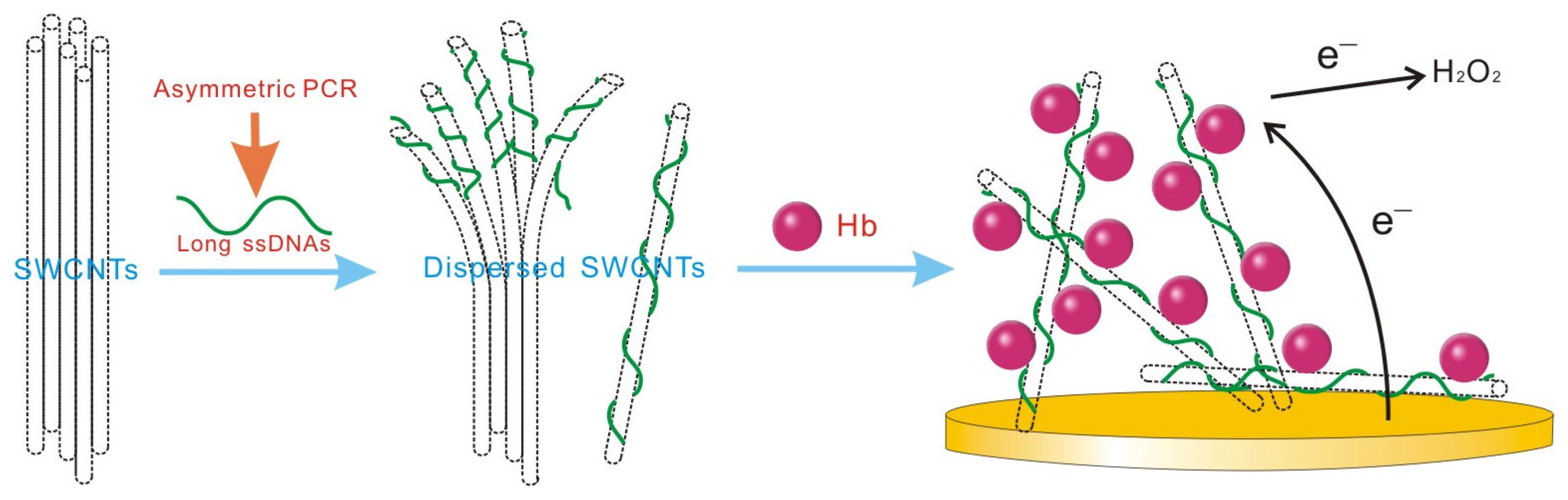
2. Results and Discussion
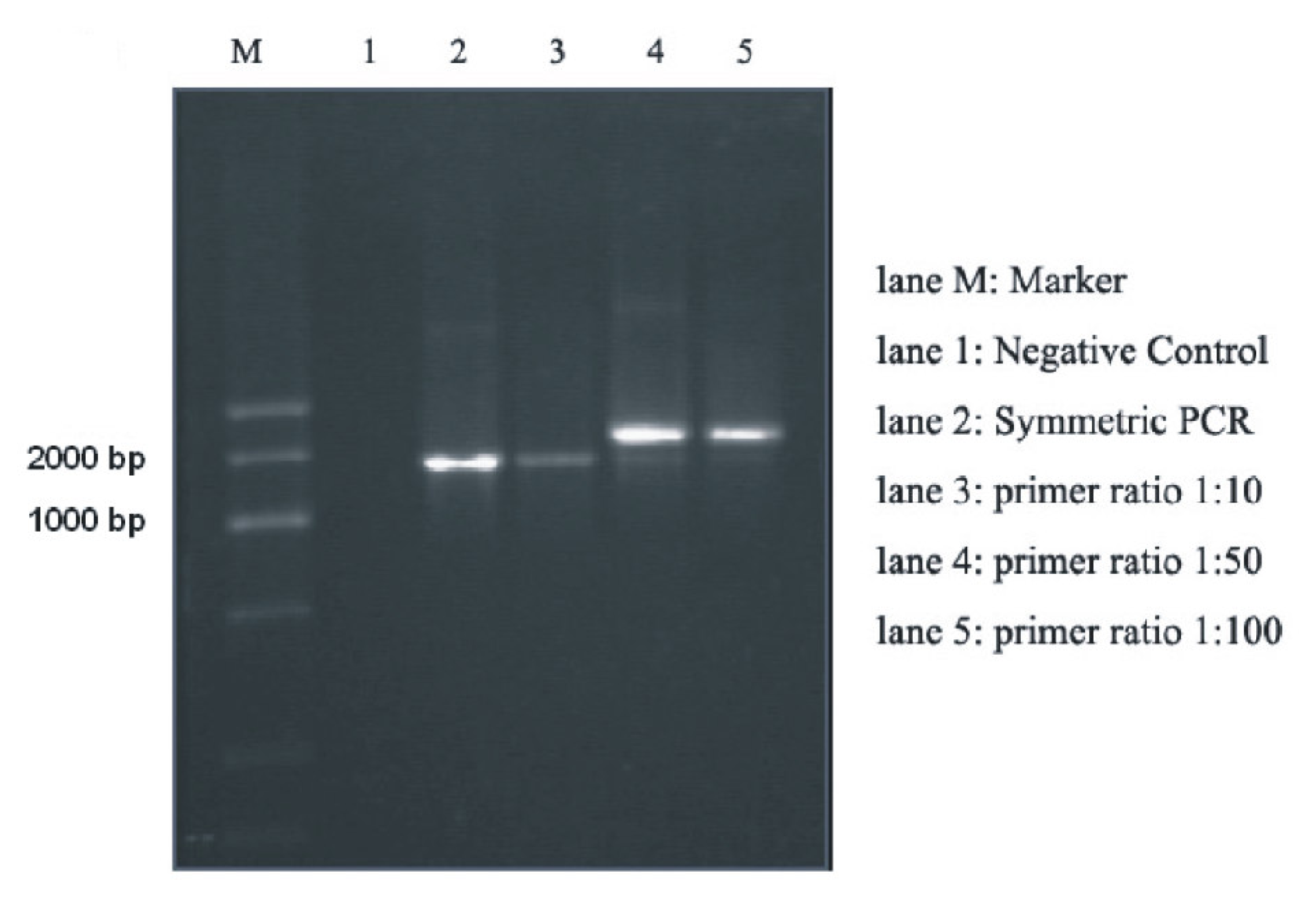
2.1. Amplification of DNA Templates Using Asymmetric PCR
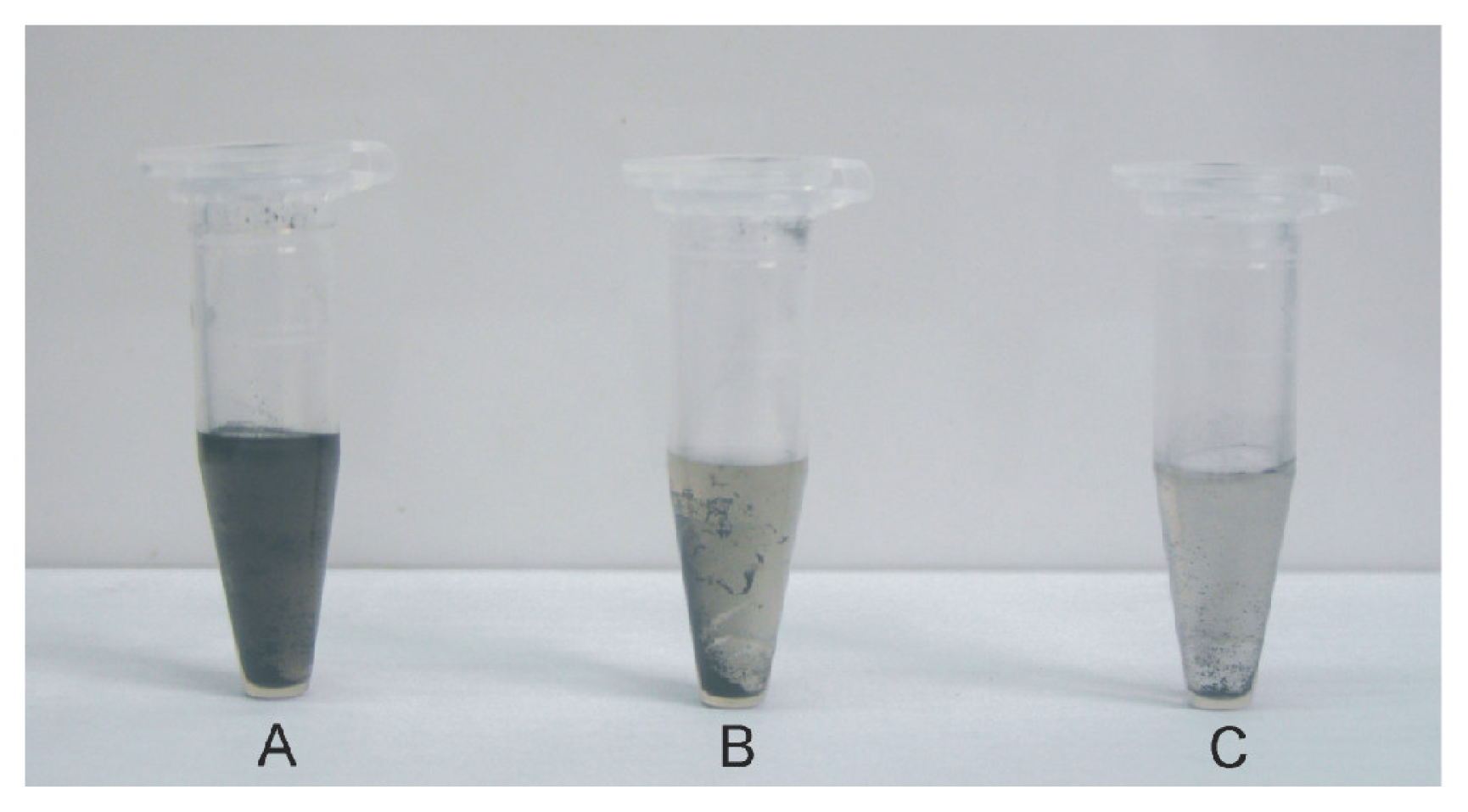
2.2. Solubilization of DNA-SWCNTs
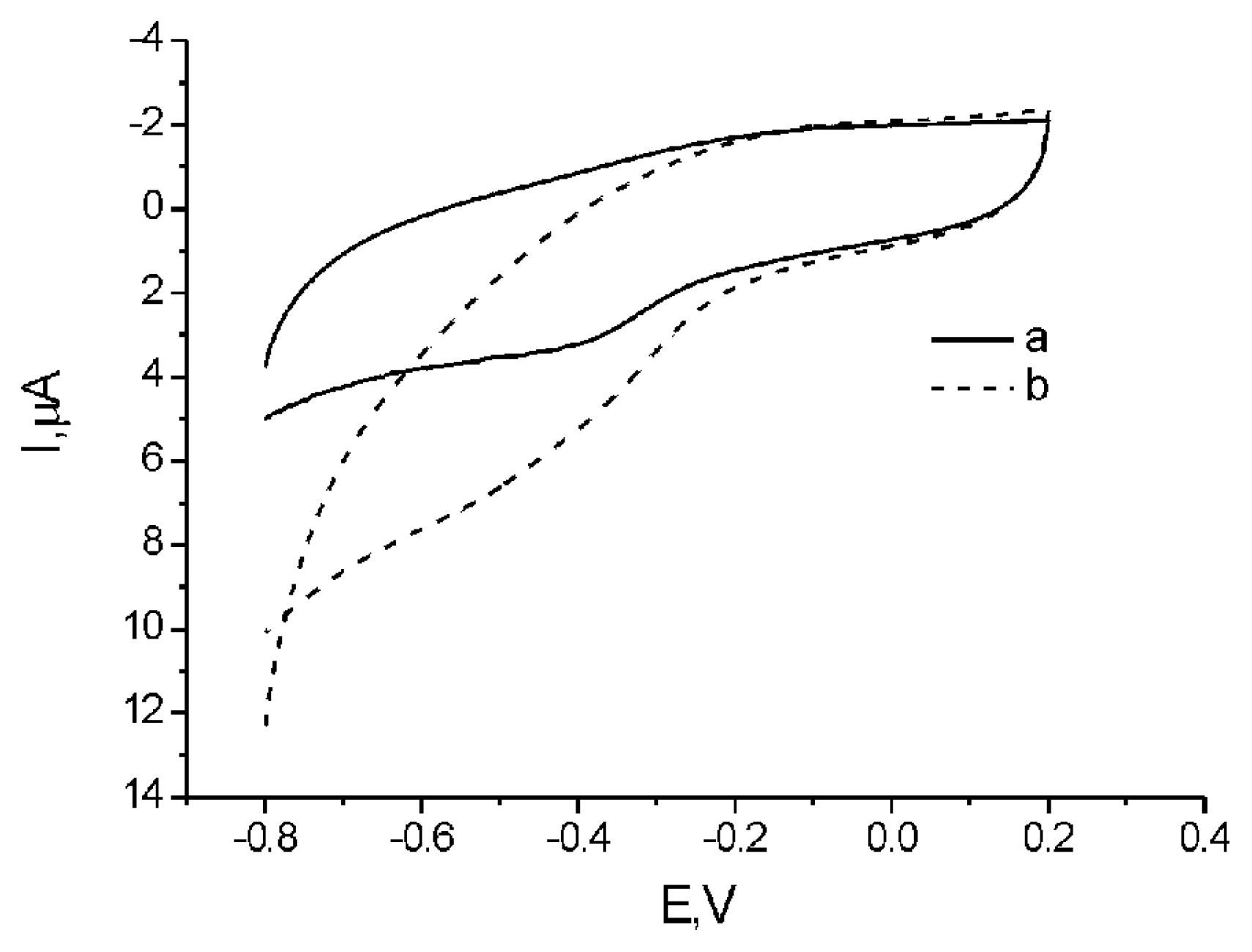
2.3. Electrochemical investigation of DNA-SWCNTs
3. Experimental Section
Acknowledgements
References and Notes
- Berber, S.; Kwon, Y.K.; Tomanek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett 2000, 84, 4613–4616. [Google Scholar]
- Wei, B.Q.; Vajtai, R.; Zhang, Z.J.; Ramanath, G.; Ajayan, P.M. Carbon nanotube-magnesium oxide cube networks. J. Nanosci. Nanotechno 2001, 1, 35–38. [Google Scholar]
- Guo, W.; Guo, Y.; Gao, H.; Zheng, Q.; Zhong, W. Energy dissipation in gigahertz oscillators from multiwalled carbon nanotubes. Phys. Rev. Lett 2003, 91, 125501. [Google Scholar]
- Giordani, S.; Bergin, S.D.; Nicolosi, V.; Lebedkin, S.; Kappes, M.M.; Blau, W.J.; Coleman, J.N. Debundling of single-walled nanotubes by dilution: observation of large populations of individual nanotubes in amide solvent dispersions. J. Phys. Chem. B 2006, 110, 15708–15718. [Google Scholar]
- Bandyopadhyaya, R.; Nativ-Roth, E.; Regev, O.; Yerushalmi-Rozen, R. Stabilization of Individual Carbon Nanotubes in Aqueous Solutions. Nano Lett 2002, 2, 25. [Google Scholar]
- Shvartzman-Cohen, R.; Levi-Kalisman, Y.; Nativ-Roth, E.; Yerushalmi-Rozen, R. Generic approach for dispersing single-walled carbon nanotubes: the strength of a weak interaction. Langmuir 2004, 20, 6085–6088. [Google Scholar]
- Dwyer, C.; Guthold, M.; Falvo, M.; Washburn, S.; Superfine, R.; Erie, D. DNA-functionalized single-walled carbon nanotubes. Nanotechnology 2002, 13, 601–604. [Google Scholar]
- O’Connell, M.; Peter, B.; Ericson, L.; Huffman, C.; Wang, Y.; Haroz, E.; Kuper, C. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett 2001, 342, 265–271. [Google Scholar]
- Zhu, J.; Yudasaka, M.; Zhang, M.; Iijima, S. Dispersing Carbon Nanotubes in Water: A Noncovalent and Nonorganic Way. J. Phys. Chem. B 2004, 108, 11317–11320. [Google Scholar]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; McLean, R.S.; Onoa, G.B.; et al. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science 2003, 302, 1545–1548. [Google Scholar]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater 2003, 2, 338–342. [Google Scholar]
- Gigliotti, B.; Sakizzie, B.; Bethune, D.S.; Shelby, R.M.; Cha, J.N. Sequence-Independent Helical Wrapping of Single-Walled Carbon Nanotubes by Long Genomic DNA. Nano Lett. 2006, 6(2), 159–164. [Google Scholar]
- Zhao, W.A.; Gao, Y.; Brook, M.A.; Li, Y.F. Wrapping single-walled carbon nanotubes with long single-stranded DNA molecules produced by rolling circle amplification. Chem. Commun. 2006, 3582–3584. [Google Scholar]
- Zhao, W.; Gao, Y.; Kandadai, S.A.; Brook, M.A.; Li, Y. DNA polymerization on gold nanoparticles through rolling circle amplification: towards novel scaffolds for three-dimensional periodic nanoassemblies. Angew. Chem.( Int. Edit.) 2006, 45, 2409–2413. [Google Scholar]
- Keren, K.; Berman, R.S.; Buchstab, E.; Sivan, U.; Braun, E. DNA-templated carbon nanotube field-effect transistor. Science 2003, 302, 1380–1382. [Google Scholar]
- Hu, C.; Zhang, Y.; Bao, G.; Zhang, Y.; Liu, M.; Wang, Z.L. DNA functionalized single-walled carbon nanotubes for electrochemical detection. J. Phys. Chem 2005, 109, 20072–20076. [Google Scholar]
- Nassar, A.E.F.; Rusling, J.F. Electron Transfer between Electrodes and Heme Proteins in Protein-DNA Films. J. Am. Chem. Soc 1996, 118, 3043–3044. [Google Scholar]
- Ryan, M.D.; Bowden, E.F.; Chambers, J.Q. Dynamic electrochemistry: methodology and application. Anal. Chem 1994, 66, 360R–427R. [Google Scholar]
- Tong, Z.Q.; Yuan, R.; Chai, Y.Q.; Chen, S.H.; Xie, Y. Amperometric biosensor for hydrogen peroxide based on Hemoglobin/DNA/Poly-2,6-pyridinediamine modified gold electrode. Thin Solid Films 2007, in press. [Google Scholar]
- Lao, R.; Wang, L.; Wan, Y.; Zhang, J.; Song, S.; Zhang, Z.; Fan, C.; Lin, H. Interactions between Cytochrome c and DNA Strands Self-Assembled at Gold Electrode. Int. J. Mol. Sci 2007, 8, 136–144. [Google Scholar]
- Zhou, Y.; Li, Z.; Hu, N.; Zeng, Y.; Rusling, J.F. Layer-by-Layer Assembly of Ultrathin Films of Hemoglobin and Clay Nanoparticles with Electrochemical and Catalytic Activity. Langmuir 2002, 18, 8573–8579. [Google Scholar]
- Liu, X.J.; Xu, Y.; Ma, X.; Li, G. A third-generation hydrogen peroxide biosensor fabricated with hemoglobin and Triton X-100. Sensor. Actuat. B 2005, 106, 284–288. [Google Scholar]
- Zhou, H.; Gan, X.; Wang, J.; Zhu, X.; Li, G. Hemoglobin-based hydrogen peroxide biosensor tuned by the photovoltaic effect of nano titanium dioxide. Anal. Chem 2005, 77, 6102–6104. [Google Scholar]
- Ma, X.; Liu, X.; Xiao, H.; Li, G. Direct electrochemistry and electrocatalysis of hemoglobin in poly-3-hydroxybutyrate membrane. Biosens. Bioelectron 2005, 20, 1836–1842. [Google Scholar]
- Fan, C.; Wang, H.; Sun, S.; Zhu, D.; Wagner, G.; Li, G. Electron-transfer reactivity and enzymatic activity of hemoglobin in a SP Sephadex membrane. Anal. Chem 2001, 73, 2850–2854. [Google Scholar]
- Huang, H.; Hu, N.; Zeng, Y.; Zhou, G. Electrochemistry and electrocatalysis with heme proteins in chitosan biopolymer films. Anal. Biochem 2002, 308, 141–151. [Google Scholar]
© 2007 by MDPI ( http://www.mdpi.org) Reproduction is permitted for noncommercial purposes.
Share and Cite
Liang, Z.; Lao, R.; Wang, J.; Liu, Y.; Wang, L.; Huang, Q.; Song, S.; Li, G.; Fan, C. Solubilization of Single-walled Carbon Nanotubes with Single- stranded DNA Generated from Asymmetric PCR. Int. J. Mol. Sci. 2007, 8, 705-713. https://doi.org/10.3390/i8070705
Liang Z, Lao R, Wang J, Liu Y, Wang L, Huang Q, Song S, Li G, Fan C. Solubilization of Single-walled Carbon Nanotubes with Single- stranded DNA Generated from Asymmetric PCR. International Journal of Molecular Sciences. 2007; 8(7):705-713. https://doi.org/10.3390/i8070705
Chicago/Turabian StyleLiang, Zhiqiang, Ruojun Lao, Jing Wang, Yongbiao Liu, Lihua Wang, Qing Huang, Shiping Song, Genxi Li, and Chunhai Fan. 2007. "Solubilization of Single-walled Carbon Nanotubes with Single- stranded DNA Generated from Asymmetric PCR" International Journal of Molecular Sciences 8, no. 7: 705-713. https://doi.org/10.3390/i8070705



