Antioxidant Activity of a Series of FluorinatedPyrano-nucleoside Analogues of N4-benzoyl Cytosine andN6-benzoyl Adenine
Abstract
:1. Introduction
2. Results and Discussion
2.1. DPPH radical scavenging activity
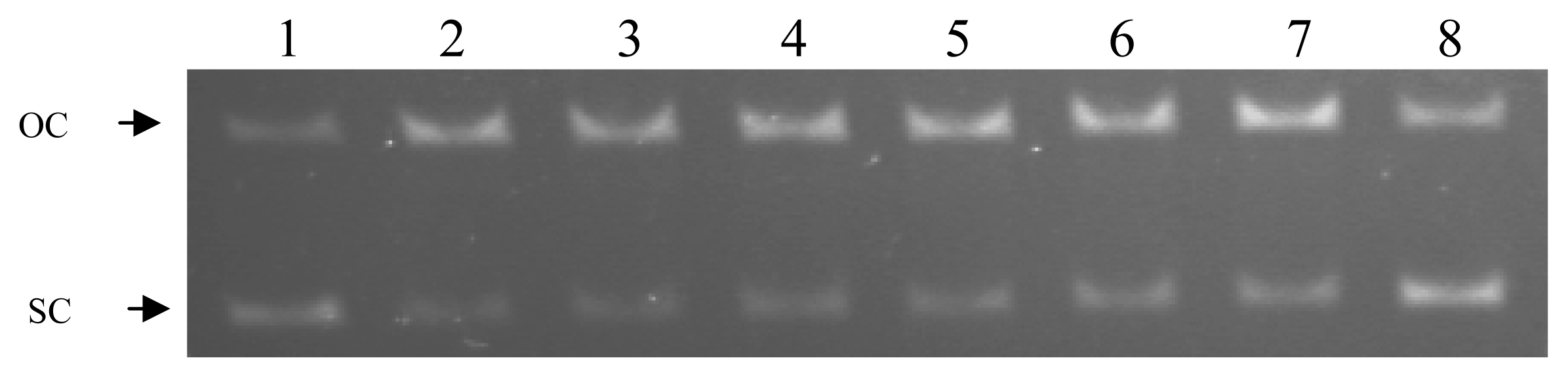
2.2. Protecting effect of the tested nucleoside analogues against peroxyl radical-induced DNA strand scission
2.3. Protecting effect of the tested nucleoside analogues against hydroxyl radical-induced DNA strand scission
2.4. Conclusions
3. Experimental Section
3.1. Chemicals
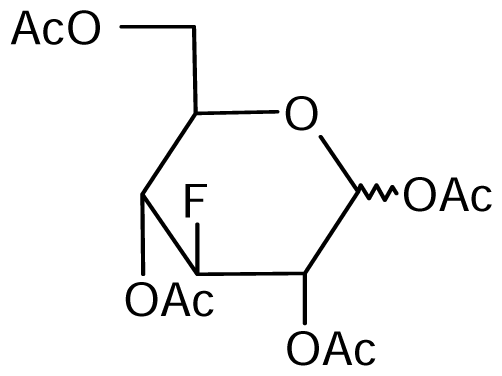
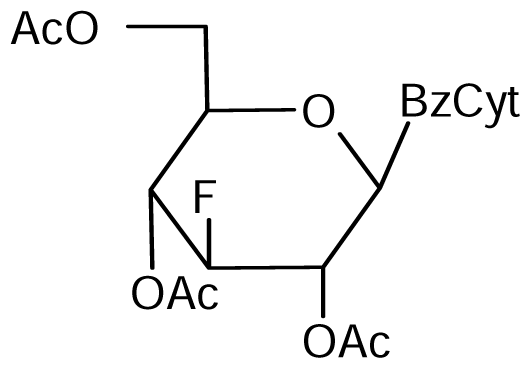
3.2. Nucleoside analogues and their Preparation
3.3. DPPH assay
3.4. Evaluation of the protecting effect of the compounds by peroxyl radical-induced DNA strand scission assay
3.5. Evaluation of the protecting effect of the compounds by hydroxyl radical-induced DNA strand scission assay
3.6. Percent inhibition of Peroxyl and Hydroxyl radical-induced DNA strand scission
3.7. Statistical analysis
| Products | % Inhibition | Products | % Inhibition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| μM | 5 | 10 | 20 | 50 | 100 | μM | 5 | 10 | 20 | 50 | 100 |
 1 | NI | NI | NI | NI | NI | 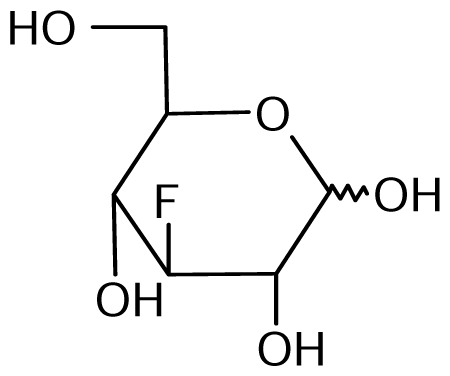 2 | NI | NI | NI | NI | NI |
 3 | NI | NI | NI | 11±1A* | 24±1* | 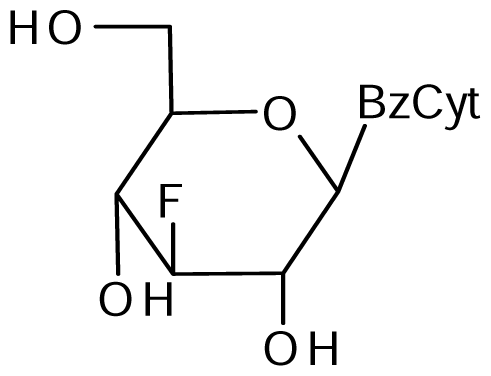 4 | NIB | NI | 10±5 | 11±1* | 27±4* |
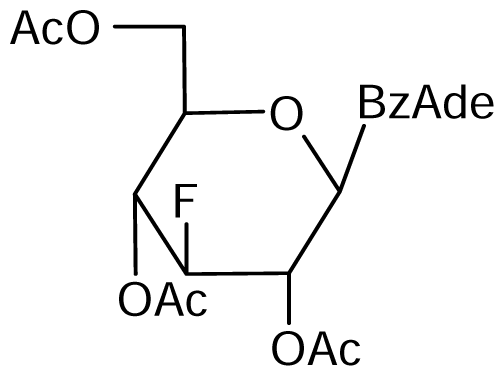 5 | NI | NI | NI | NI | NI | 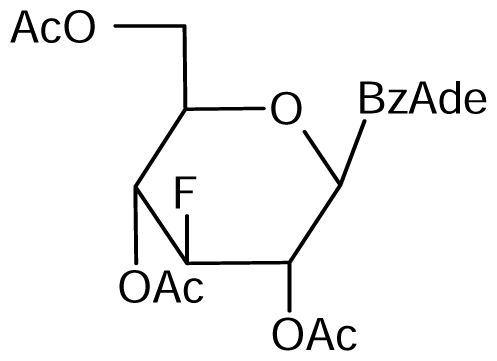 6 | NI | NI | NI | NI | NI |
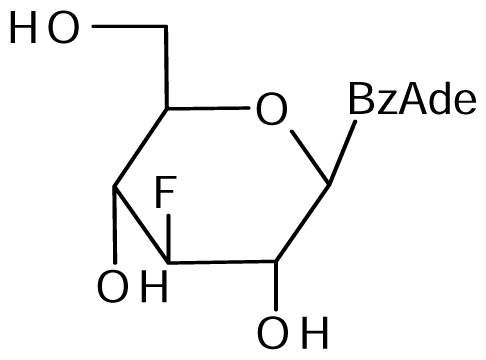 7 | NI | NI | 7±2 | 7±3 | 11±3 | 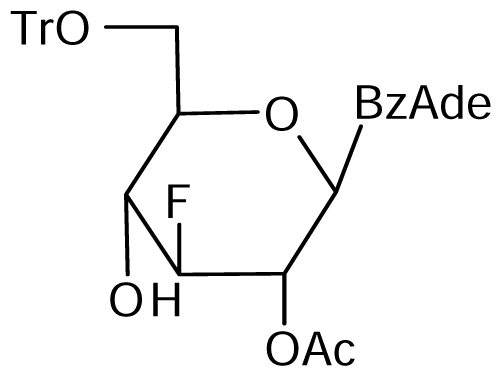 8 | NI | NI | NI | 16±4 | 20±2* |
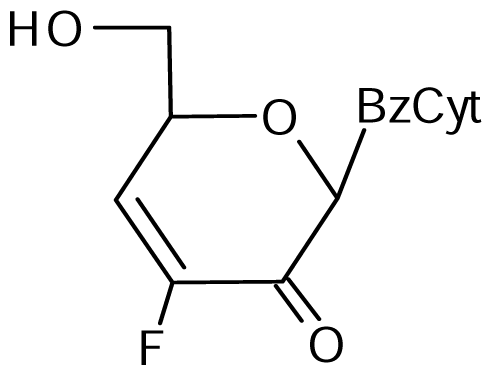 9 | NI | NI | NI | 16±5 | 27±3* | 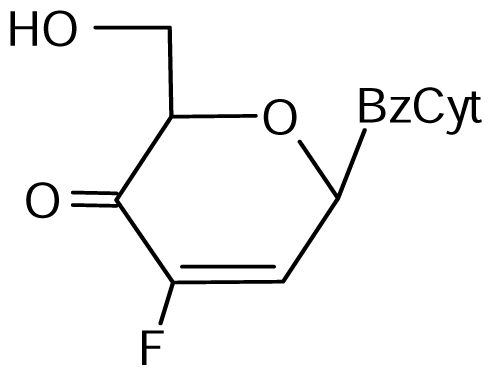 10 | 9±3 | 18±1* | 19±1* | 25±3* | 26±2* |
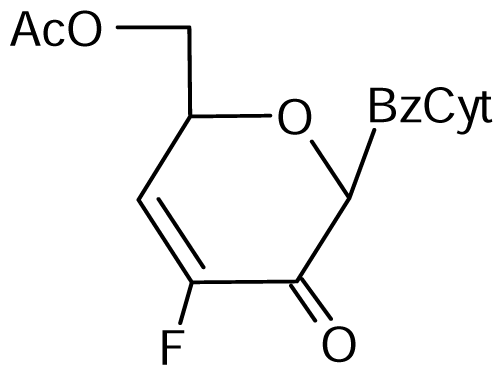 11 | NI | NI | 8±3 | 20±2* | 37±1* |  12 | NI | NI | NI | NI | NI |
 13 | NI | NI | NI | NI | NI | ||||||
References and Notes
- Zhou, W.; Gumina, G.; Chong, Y.; Wang, J.; Shinazi, R.F.; Chu, C.K. Synthesis, Structure-Activity Relationships, and Drug Resistance of β-d-3′-Fluoro-2′,3′-Unsaturated Nucleosides as Anti-HIV Agents. J. Med. Chem 2004, 47, 3399–3408. [Google Scholar]
- Perigaud, C.; Gosselin, G.; Imbach, J.L. Nucleosides Analogues as Chemotherapeutic Agents: A Review. Nucleosides Nucleotides 1992, 11, 903–945. [Google Scholar]
- Robins, R.K.; Kini, G.D. Purines and Purine Nucleoside Analogues as Antitumor Agents. In The Chemistry of Antitumor Agents; Wilman, D.E.V., Ed.; Chapman and Hall: New York, 1990; pp. 299–321. [Google Scholar]
- MacCoss, M.; Robins, M.J. Anticancer Pyrimidines, Pyrimidine Nucleosides and Prodrugs. In the chemistry of Antitumor Agents; Wilman, D.E.V., Ed.; Chapman and Hall: New York, 1990; pp. 261–298. [Google Scholar]
- Verheggen, I.; Van Aerschot, A.; Toppet, S.; Snoeck, R.; Janssen, G.; Balzarini, J.; De Clercq, E.; Herdewijn, P. Synthesis and Antiherpes Virus Activity of 1,5-Anhydrohexitol Nucleosides. J. Med. Chem 1993, 36, 2033–2040. [Google Scholar]
- Verheggen, I.; Van Aerschot, A.; Van Meervelt, L.; Rozenski, J.; Wiebe, L.; Snoeck, R.; Andrei, G.; Balzarini, J.; Claes, P.; De Clercq, E.; Herdewijn, P. Synthesis, Biological Evaluation and Structure Analysis of New 1,5-Anhydrohexitol Nucleosides. J. Med. Chem 1995, 38, 826–835. [Google Scholar]
- Ostrowski, T.; Wroblowski, B.; Busson, R.; Rozenski, J.; De Clercq, E.; Bennet, M.S.; Champness, J.N.; Summers, W.C.; Sanderson, M.R.; Herdewijn, P. 5-Substituted Pyrimidines with a 1,5-Anhydro-2,3-Dideoxy-d-Arabino-Hexitol Moiety at N-1: Synthesis, Antiviral Activity, Conformational Analysis, and Interaction with Viral Thymidine Kinase. J. Med. Chem 1998, 41, 4343–4353. [Google Scholar]
- Maurinsh, Y.; Schraml, J.; De Winter, H.; Blaton, N.; Peeters, O.; Lescrinier, E.; Rozenski, J.; Van Aerschot, A.; De Clercq, E.; Busson, R.; Herdewijn, P. Synthesis and Conformational Study of 3-Hydroxy-4-(Hydroxymethyl)-1-Cyclohexanyl Purines and Pyrimidines. J. Org. Chem 1997, 62, 2861–2871. [Google Scholar]
- Haouz, A.; Vanheusden, V.; Munier-Lechman, H.; Froeyen, M.; Herdewijn, P.; Van Galenbergh, S.; Delarue, M. Enzymatic and Structural Analysis of Inhibitors Designed Against Mycobacterium Tuberculosis Thymidylate Kinase. New Insights into the Phosphoryl Transfer Mechanism. J. Biol. Chem 2003, 278, 4963–4971. [Google Scholar]
- Vastmans, K.; Pochet, S.; Peys, A.; Kerremans, L.; Van Aerschot, A.; Hendrix, C.; Marliere, P.; Herdewijn, P. Enzymatic Incorporation in DNA of 1,5-Anhydrohexitol Nucleotides. Biochemistry 2000, 39, 12757–12765. [Google Scholar]
- Vastmans, K.; Froeyen, M.; Kerremans, L.; Pochet, S.; Herdewijn, P. Reverse Transcriptase Incorporation of 1,5-Anhydrohexitol Nucleotides. Nucleic Acids Res 2001, 29, 3154–3163. [Google Scholar]
- Paterson, J.; Uriel, C.; Egron, M.J.; Herscovici, J.; Antonakis, K.; Alaoui, M. Antiproliferative and Apoptotic Activities of Ketonucleosides and Keto-C-Glycosides against Non-Small-Cell Lung Cancer Cells with Intrinsic Drug Resistance. Antimicrob. Agents Chemother 1998, 42, 779–784. [Google Scholar]
- Alaoui, M.; Lasne, C.; Antonakis, K.; Chouroulinkov, I. Absence of Genotoxic Effects in Cells Exposed to Four Ketonucleoside Derivatives. Mutagenesis 1986, 1, 411–417. [Google Scholar]
- Alaoui, M.; Egron, M.J.; Bessodes, M.; Antonakis, K.; Chouroulinkov, I. Relationship Between The Structure and Cytotoxic Activity of New Unsaturated Ketonucleosides Tested on Eight Cell Lines. Eur. J. Med. Chem 1987, 22, 305–310. [Google Scholar]
- Egron, M.J.; Komiotis, D.; Dorange, I.; Herscovici, J.; Antonakis, K. New Short Route to Unsaturated Fluoroketonucleosides: Case of 5-Fluoro-1-(6-O-Acetyl-3,4-Di-deoxy-3-Fluoro-β-d-Glycero- Hex-3-Eno-Pyranos-2-Ulosyl) Uracil. Nucleosides, Nucleotides, and Nucleic Acids 2005, 24, 243–246. [Google Scholar]
- Halmos, T.; Komiotis, D.; Antonakis, K. Cancer Oriented Ketonucleosides: Synthesis of 7-[6-O-(5-Carboxypentyl)-3.4-Dideoxy-and 3,4-Dideoxy-6-O-(6-Hydroxyexyl)-β-d-Glycero-Hex-3-Enopyranosyl-2-ulose]Theophyllines and Their Coupling with Cancer-Specific Proteins. Carbohydr. Res 1986, 156, 256–263. [Google Scholar]
- Antonakis, K.; Chouroulinkov, I. Studies on the Inhibition of the Growth of KB Cells by 9- and 7-(Ketonucleosides). Biochem. Pharmacol 1974, 23, 2095–2100. [Google Scholar]
- Halmos, T.; Cardon, A.; Antonakis, K. Interactions of Cytostatic Unsaturated Ketonucleosides with Sulfhydryl Containing Cell Constituents. Chem. Biol. Interact 1983, 46, 11–29. [Google Scholar]
- Antonakis, K.; Halmos, T.; Bach, J.; Chouroulinkov, I. Antitumor Activity of Ketonucleosides. Unsaturated Ketohexosyl Purines. Eur. J. Med. Chem 1980, 15, 237–240. [Google Scholar]
- Chouroulinkov, I.; Antonakis, K. Antitumor Effects of Keto-Nucleosides. Study of the Antitumor Activity of Y(3′-O-Acetyl-4′,6′-Dideoxy-Beta-l-Glycero-Hex-3′-Eno-pyranosyl)Theophylline and 1(4′-Keto-2′,3′-O-Isopropylidene-Alpha-L-“Rhammosyl” O Thymine Against L1210 Leukemia in Mice. C R Acad. Sci. Hebd Seances Acad. Sci. D 1977, 285, 1021–1024. [Google Scholar]
- Antonakis, K. Synthesis of Nucleoside Analogues. Chimia 1975, 29, 59. [Google Scholar]
- Antonakis, K.; Egron, M.J. Unsaturated Ketonucleosides. The Synthesis and Properties of 7-(3-O-Acetyl- 4,6-Dideoxy-β-l-Glycero-Hex-3 Enopyranosylulose)Theophylline. Carbohydr. Res 1973, 27, 468–470. [Google Scholar]
- Egron, M.J.; Leclercq, F.; Antonakis, K.; Bennani-Baiti, I.; Frayssinet, C. Synthesis and Antineoplastic Properties of 3′-Deoxy-3′-Fluoroketonucleoside Derivatives. Correlations Between Structure and Biological Activity. Carbohydr. Res 1993, 248, 143–150. [Google Scholar]
- Van Aerschot, A.; Herdewijn, P.; Balzarini, J.; Pawels, R.; De Clerq, E. 3′-Fluoro-2′,3′-Dideoxy-5-Chlorouridine: Most Selective Anti-HIV-1 Agent Among a Series of New 2′- and 3′-Fluorinated 2′,3′-Dideoxynucleoside Analogues. J. Med. Chem 1989, 32, 1743–1749. [Google Scholar]
- Blandin, M.; Son, T.D.; Catlin, J.C.; Guschlbauer, W. Nucleoside conformations. 16. Nuclear Magnetic Resonance and Circular Dichroism Studies on Pyrimidine-2′-Fluoro-2′-Deoxyribonucleosides. Biochim. Biophys. Acta 1974, 361, 249–253. [Google Scholar]
- Morton, G.O.; Lancaster, J.E.; Van Lear, G.E.; Fulmor, W.; Meyer, W.E. The Structure of Nucleocidin. 3. (A New Structure). J. Amer. Chem. Soc 1969, 91, 1535–1537. [Google Scholar]
- Lipnick, R.L.; Fissekis, J.D. A Comparative Conformational Study of Certain 2′-Deoxy-2′-Fluoro-Arabinofuranosylcytosine Nucleosides. Biochim. Biophys. Acta 1980, 608, 96–102. [Google Scholar]
- Manta, S.; Agelis, G.; Botic, T.; Cencic, A.; Komiotis, D. Fluoro-Ketopyranosyl Nucleosides: Synthesis and Biological Evaluation of 3-Fluoro-2-Keto-β-d-Glucopyranosyl Derivatives of N4-Benzoyl Cytosine. Bioorg. Med. Chem 2007, 15, 980–987. [Google Scholar]
- Manta, S.; Agelis, G.; Botic, T.; Cencic, A.; Komiotis, D. Unsaturated Fluoro-Ketopyranosyl Nucleosides: Synthesis and Biological Evaluation of 3-Fluoro-4-Keto-β-d-Glucopyranosyl Derivatives of N4-Benzoyl Cytosine and N6-Benzoyl Adenine. Eur. J. Med. Chem. in press.
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem 2005, 53, 4290–4302. [Google Scholar]
- Mylonas, C.; Kouretas, D. Lipid Peroxidation and Tissue Damage. In Vivo 1999, 13, 295–310. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol 1995, 28, 25–30. [Google Scholar]
- Chang, S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.L.; Yang, N.S.; Shyur, L.F. Antioxidant Activity of Extracts from Acacia confusa Bark and Heartwood. J. Agric. Food Chem 2001, 49, 3420–3424. [Google Scholar]
- Spanou, C.; Stagos, D.; Tousias, L.; Angelis, A.; Aligiannis, N.; Skaltsounis, A.L.; Kouretas, D. Assessment of Antioxidant Activity of Extracts from Unique Greek Varieties of Leguminosae Family Plants Using in vitro Assays. Anticancer Research. submitted.
- Keum, Y.S.; Park, K.K.; Lee, J.M.; Chun, K.S.; Park, J.H.; Lee, S.K.; Kwon, H.; Surch, Y. Antioxidant and Anti-tumor Promoting Activities of the Methanol Extract of the Heat-Processed Ginseng. Cancer Lett 2000, 150, 41–48. [Google Scholar]
© 2007 by MDPI ( http://www.mdpi.org) Reproduction is permitted for noncommercial purposes.
Share and Cite
Spanou, C.; Manta, S.; Komiotis, D.; Dervishi, A.; Kouretas, D. Antioxidant Activity of a Series of FluorinatedPyrano-nucleoside Analogues of N4-benzoyl Cytosine andN6-benzoyl Adenine. Int. J. Mol. Sci. 2007, 8, 695-704. https://doi.org/10.3390/i8070695
Spanou C, Manta S, Komiotis D, Dervishi A, Kouretas D. Antioxidant Activity of a Series of FluorinatedPyrano-nucleoside Analogues of N4-benzoyl Cytosine andN6-benzoyl Adenine. International Journal of Molecular Sciences. 2007; 8(7):695-704. https://doi.org/10.3390/i8070695
Chicago/Turabian StyleSpanou, Chrysoula, Stella Manta, Dimitri Komiotis, Albiona Dervishi, and Demetrios Kouretas. 2007. "Antioxidant Activity of a Series of FluorinatedPyrano-nucleoside Analogues of N4-benzoyl Cytosine andN6-benzoyl Adenine" International Journal of Molecular Sciences 8, no. 7: 695-704. https://doi.org/10.3390/i8070695




