iii ) Simultaneous Cooling Method
The reaction mixture solved in the properly choose solvent was subjected to microwave irradiation in a reaction vessel provided with a cooling mantle. During irradiation, the circulation of the cooling agent ensures the control of the reaction temperature and avoids the super heating of the solvent. Work-up of the reaction product was similar to the methods indicated above.
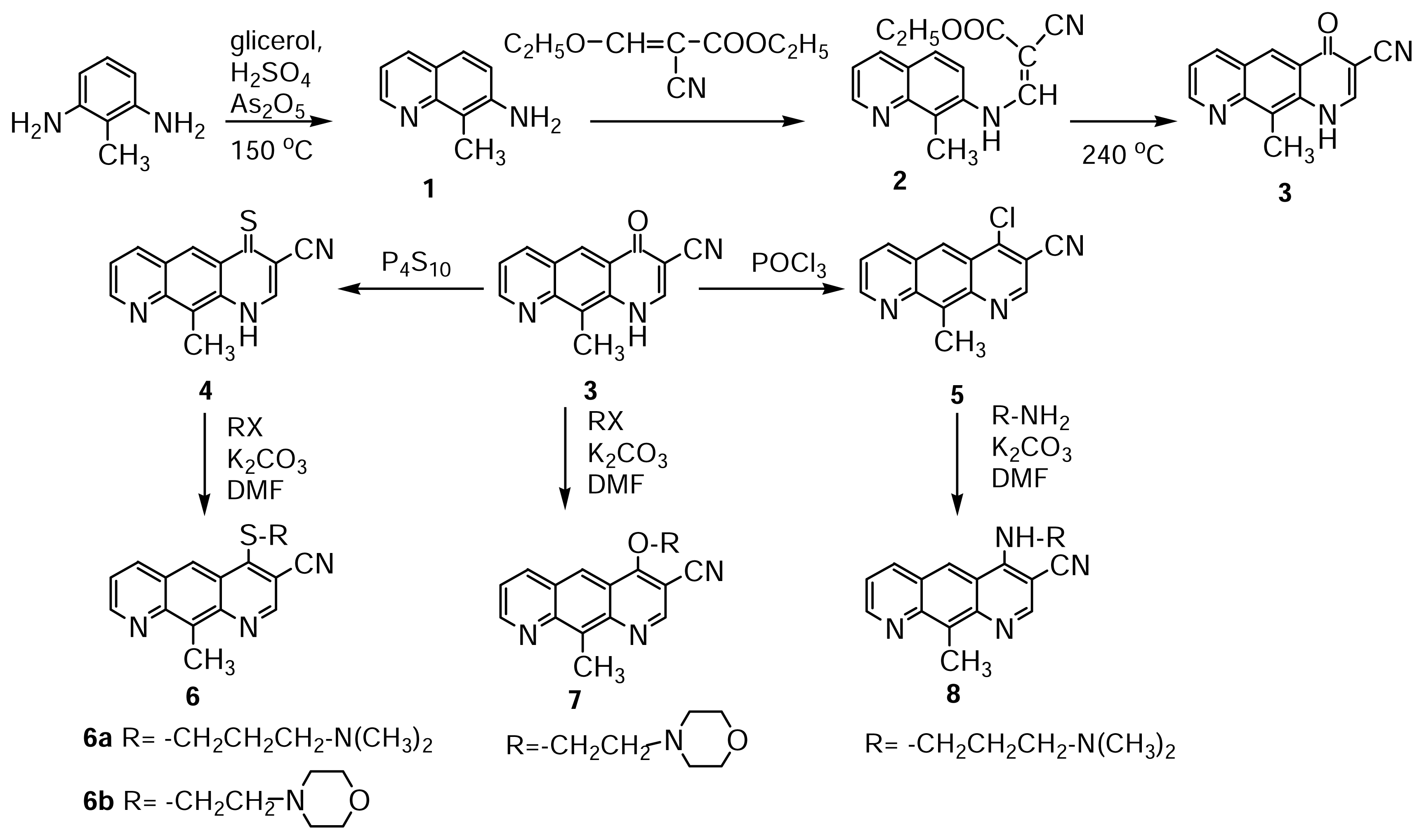
7-Amino-8-methyl-quinoline 1
A mixture of 4 mmol 2,6-diaminotoluene (0.5g), glycerol (2.5 mL), arsenic(V) oxide (2.1g) and sulfuric acid (conc. 4.2 mL) was subjected to microwave irradiation according to procedure ii). After cooling at room temperature, the reaction mixture was poured into an ice-water mixture (15 mL) and alkalinized at pH=9-10. The precipitate obtained was filtered, washed with cold water and crystallized from water; 0.2 g of yellow powder (m.p.=128 °C) was obtained.
NMR (400MHz, CHCl3-d1): δH (ppm) 2.59 (s, 3H), 4.0 (s, -NH2), 7.0 (d, 1H, J=8.8 Hz), 7.14 (dd, 1H, J=4.4 Hz, J=8.2 Hz), 7.49 (d, 1H, J = 8.8 Hz), 7.9 (dd, 1H, J=2 Hz, J=8.2 Hz), 8.8 (dd, 1H, J=2 Hz, J=4.4 Hz). δC (ppm) 10.25; 115.03; 117.32; 118.38; 122.56; 126.17; 136.06; 144.79; 148.26; 149.61.
(8-Methyl-quinoline-7-aminomethylene)ethyl cyanoacetate 2
A mixture of 3.1 mmol 7-amino-8-methyl-quinoline (0.5g,) and 9.3 mmol ethyl-(etoxymethylene)-cyanoacetate (1.5g,) was subjected to microwave irradiation. The reaction product (0.8g of white powder, m.p.=206 °C) was obtained according to procedure i) using 10 g silica gel solid support. Crystallization from ethanol gave the pure product.
NMR (400MHz, DMSO-d6): δH (ppm): 1.24 (t, 3H, J=7 Hz, -CH3), 2.84 (s, 3H, -CH3), 4.35 (q, 2H, J=7), 7.39 (dd, 1H, J=4 Hz, J=8.2 Hz), 7.42 (d, 1H, J= 8.8Hz), 7.77 (d, 1H, J=8.8 Hz), 8.01 (d, 1H, J=13 Hz), 8.12 (dd, 1H, J=1.6 Hz, J=8.2 Hz), 8.96 (dd, 1H, J=1.6 Hz, J=4 Hz), 11,31 (d, 1H, J=13 Hz, NH). δC (ppm): 10.92; 14.33; 61.44; 76.3 (CN); 115,00; 117.81; 120.60; 124.43; 125.97; 127.51; 136.33; 136.43; 147.54, 150.68; 151.81; 167.74.
10-methyl-4-oxo-pyrido[3,2-g]quinolin-3-carbonitrile 3
1.7 mmol (8-methyl-quinoline-7-aminomethylene)-ethylcyanoacetate (0.5g,) were subjected to microwave irradiation according to procedure i), using 10g silica gel solid support and 10g clay (acidic bentonite) respectively. The reaction product (0.3g /0.35g of white powder, m.p.= 292 °C) was obtained by pouring the dimethylformamide extract in water, followed by filtration of the precipitate.
NMR (400MHz, DMSO-d6): δH (ppm): 2.93 (s, 1H), 7.57 (dd, 1H, J=4 Hz, J=8.4 Hz), 8.59 (dd, 1H, J=1.6 Hz, J=8.4 Hz), 8.65 (s, 1H), 8.71 (s, 1H), 9.06 (dd, 1H, J=1.6 Hz, J=4 Hz). δC (ppm): 11.19; 91.41 (-CN); 116.91; 121.56; 123.94; 124.52; 124.96; 125.01; 136.26; 138.43; 146.49; 148.51; 153.18; 175.77.
10-methyl-4-thioxo-pyrido[3,2-g]quinolin-3-carbonitrile 4
A mixture of 2.5 mmol 3 (0.6g,) and 1.1 mmol tetra-phosphorus decasulfide P4S10 (0.5g,) was subjected to microwave irradiation according to procedure i), using 1g silica gel solid support and 1g clay (acidic bentonite) respectively. The reaction product (0.6g /0.4g of orange powder, m.p.= 196 °C with decompn.) was obtained by pouring the dimethylformamide extract in water, followed by filtration of the precipitate.
NMR (400MHz, DMSO-d6): δH (ppm): 2.99 (s, 3H), 7.6 (dd, 1H, J=6.4 Hz, J=7.2 Hz), 8.60 (s, 1H), 8.64 (dd, 1H, J=7.2 Hz, J=1.8 Hz), 9.10 (dd, 1H, J=6.4 Hz, J=1.8 Hz), 9.11 (s, 1H). δC (ppm): 11.2; 106.1; 114.3; 115.9; 116.2; 120.6; 126.8; 131.4; 134.9; 147.2; 148.8; 156.4; 159.8; 228.6.
4-Chloro-10-methyl-pyrido[3,2-g]quinolin-3-carbonitrile 5
A mixture of 2.1 mmol 3 (0.5g) and phosphorous oxychloride (0.2 mL,) was subjected to microwave irradiation according to procedure i), using 1g silica gel solid support. 0.5 g of green-yellow powder, m.p.= 264 °C (with decompn.) were obtained.
NMR (400MHz, DMSO-d6): δH (ppm): 3.02 (s, 3H), 7.38 (dd, 1H, J=8.4 Hz, J= 4Hz), 8.34 (s, 1H), 8.44 (dd, 1H, J=8.4 Hz, J=1,2 Hz), 8.60 (s, 1H), 8.93 (dd, 1H, J=4Hz, J=1.2 Hz). δC (ppm): 12.2; 104.3; 116.4; 120.8; 128.3; 128.4; 126.5; 135.2; 136.7; 137.5; 145.4; 149.5; 149.9; 154.8.
4-(3-N,N-dimethylamino-propylthio)-10-methyl-pyrido[3,2-g]quinolin-3-carbonitrile 6a
A mixture of 1.4 mmol 4 (0.5g), 5 mmol N,N-dimethylaminopropyl chloride hydrochloride (0.8g) and anhydrous K2CO3 (0.6g), was subjected to microwave irradiation according to procedure i), using 1g silica gel solid support. 0.4g of yellow powder, m.p.=74 °C were obtained.
NMR (400MHz, CHCl3-d1): δH (ppm): 1.86 (m, 2H), 2.19 (s, 6H), 2.44 (t, 2H, J=7.2 Hz), 3.37 (s, 3H), 3.43 (t, 2H, J =7.2 Hz), 7.53 (dd, 1H, J=8.4 Hz, J=3.6 Hz), 8.98 (s, 1H), 8.41 (dd, 1H, J=8.4 Hz, J=1,2 Hz), 9.0 (s, 1H), 9.16 (dd, 1H, J=3.6 Hz, J=1.6 Hz). δC (ppm): 12.47; 27.88; 34.25, 45.33; 57.75; 109.90; 117.25, 121.98; 124.24; 124.58; 127.35; 137.46; 146.44; 149.61, 138.21; 144.10; 152.55; 153.2.
4-(morpholino-ethylthio)-10-methyl-pyrido[3,2-g]quinolin-3-carbonitrile 6b
A mixture of 1.4 mmol 4 (0.5g,), 4 mmol 2-chloroethyl morpholine hydrochloride (0.9g) and anhydrous K2CO3 (0.6g), was subjected to microwave irradiation according to procedure i), using 1g silica gel solid support. 0.6g of yellow powder, m.p.=159 °C were obtained.
NMR (400MHz, CHCl3-d1): δH (ppm): 2.39 (t, 4H), 2.72 (t, 2H. J=6.4 Hz), 3.37 (s, 3H), 3.48 (s, 4H), 3.50 (t, 2H, J=5.2 Hz), 7.53 (dd, 1H, J=8.4 Hz, J=3.6 Hz), 8.97 (s, 1H), 8.40 (dd, 1H, J=8.4 Hz, J=1,2 Hz), 9.03 (s, 1H), 9.16 (dd, 1H, J=3.6 Hz, J=1.6 Hz). δC (ppm): 12.47 (-CH3); 33.56; 58.29; 53.24; 66.68; 110.02 (-CN); 152.57; 117.30; 137.40; 122.0; 124.31; 127.28; 153.90; 125.73; 146.39; 148.83, 138.16; 144.06;
4-(morpholino-ethyloxy)-10-methyl-pyrido[3,2-g]quinolin-3-carbonitrile 7
A mixture of 2.1 mmol 3 (0.5g,), 2.1 mmol 2-chloroethyl morpholine hydrochloride (0.4 g,) and anhydrous K2CO3 (0.6g), was subjected to microwave irradiation according to procedure i), using 1g silica gel solid support. 0.6g of yellow powder, m.p.=173 °C were obtained.
NMR (400MHz, CHCl3-d1): δH (ppm): 2.32 (t, 4H), 2.51 (t, 2H), 3.10 (s, 3H), 3.54 (t, 4H), 4.50 (t, 2H), 7.52 (dd, 1H, J=8.4 Hz, J=4.2 Hz), 8.25 (s, 1H), 8.37 (dd, 1H, J=8.4 Hz, J=1,8 Hz), 8.86 (s, 1H), 9.09 (dd, 1H, J=3.9 Hz, J=2.1 Hz),. δC (ppm) 12.6; 53.2; 54.2; 66.6; 66.4; 112.2; 117.30; 122.0; 124.31; 125.73; 127.28; 137.40; 138.16; 144.06; 146.39; 148.83, 152.57; 153.90.
4-(1-propylamino)-10-methyl-pyrido[3,2-g]quinolin-3-carbonitrile 8
A mixture of 1.9 mmol 5 (0.5g,), 3.5 mmol N,N-dimethyl-1,3-propanediamine hydrochloride (0.5 g) and anhydrous K2CO3 (0.6g), was subjected to microwave irradiation according to procedure i), using 1g silica gel solid support. 0.4g of yellow powder, m.p.=158 °C were obtained.
NMR (400MHz, CHCl3-d1): δH (ppm): 2.42 (t, 3H), 2.77 (m, 2H), 3.29 (s, 3H), 4.04 (t, 2H), 4.05 (t, 1H), 7.44 (dd, 1H, J=5.4 Hz, J=0.9 Hz), 8.16 (s, 1H), 8.32 (dd, 1H, J=8.1 Hz, J=6,6 Hz), 8.67 (s, 1H), 9.10 (dd, 1H, J=2.7 Hz; J=1.8 Hz). δC (ppm): 12.6; 26.6; 44.2; 42.8; 56.4; 91.41; 116.91; 121.56; 123.94; 124.52; 124.96; 125.01; 136.26; 138.43; 146.49; 148.51; 153.18; 155.77.
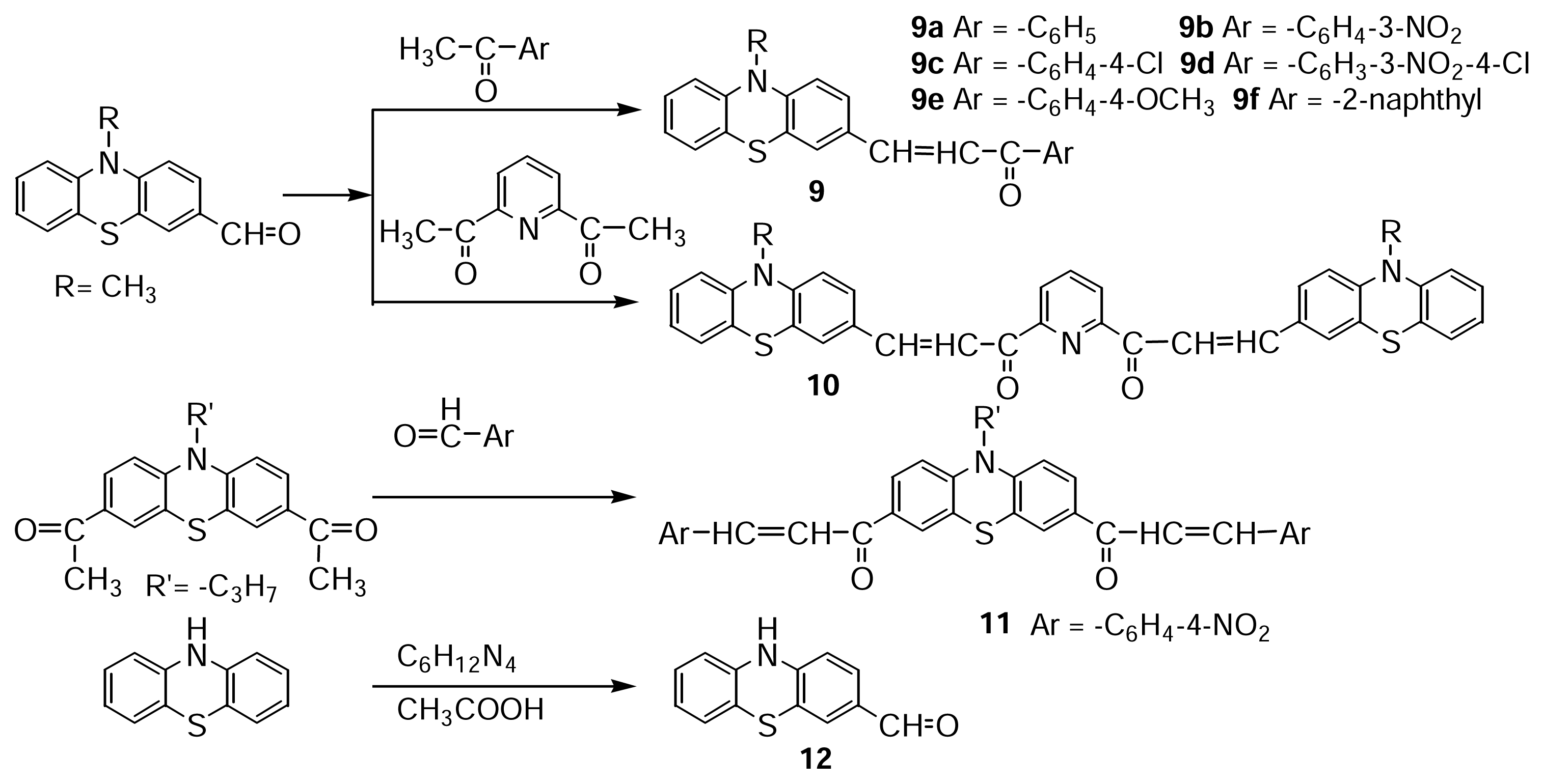
(E)-1-(10-methyl-10Hphenothiazin-3-yl)-3-phenyl-propenone 9a
A mixture of 0.3 mmol 10-methyl-3-formylphenothiazine (0.073 g), 0.3 mmol acetophenone (0.036 g) and NaOH catalyst was subjected to microwave irradiation. The reaction product (yellow powder, m.p.=147 °C) was obtained according to procedure i) 0.08 g reaction product using 1 g silica gel solid support, or according to procedure iii) 0.07 g reaction product using 50 mL ethanol solvent.
NMR (400MHz, CHCl3-d1): δH(ppm): 3.41 (s, 3H), 6.80 (d, 1H J=8.Hz), 6.83 (d, 1H J=8.5Hz), 6.96 (t, 1H, J=7.6Hz, J=7.4Hz), 7.14 (dd, 1H, J=7.4Hz, J=1.4Hz), 7.18 (m, 1H), 7.40 (d, 1H, J=15.6Hz), 7.42 (dd, 2H, J=8Hz, J=1.8Hz), 7.50 (t, 2H, J=7.4Hz, J=7.8Hz), 7.58 (t, 1H), 7.71 (d, 1H, J=15.6Hz), 8.00 (dd, 2H J=7.8Hz, J=1.2Hz). δC (ppm): 35.57, 114.09, 114.44, 119.92, 122.61, 123.99, 126.28, 127.27, 127.68, 128.44, 128.60, 129.11, 129.33, 132.63, 138.45, 143.80, 144.75, 147.86, 190.36.
(E)-1-(10-methyl-10H phenothiazin-3-yl)-3-(3-nitrophenyl)-propenone 9b
A mixture of 0.15 mmol 10-methyl-3-formylphenothiazine (0.037 g), 0.15 mmol 3-nitro-acetophenone (0.026 g) and NaOH catalyst was subjected to microwave irradiation. The reaction product (orange powder, m.p. 180 °C) was obtained according to procedure i) 0.04 g reaction product using 1 g silica gel solid support, or according to procedure iii) 0.05 g reaction product using 50 mL ethanol solvent.
NMR (400MHz, CHCl3-d1): δH (ppm): 3.42 (s, 3H); 6.84, (m. 2H), 6.97, (m, 1H), 7.14 (dd, 1H), 7.19 (m, 1H), 7.39, (d, 1H), 7.44 (m, 2H), 7.70, (t, 1H), 7.79 (d, 1H), 8.34 (m, 1H), 8.42, (m, 1H); 8.82, (t, 1H). δC (ppm): 35.63, 114.13, 114.54, 118.27, 122.48, 123.19, 123.27, 124.14, 126.57, 126.89, 127.29, 127.74, 128.72, 129.55, 129.85, 134.05, 139.81, 144.53, 145.72, 148.40, 148.44, 187.69.
(E)-1-(10-methyl-10H phenothiazin-3-yl)-3-(3-nitrophenyl)-propenone 9c
A mixture of 0.15 mmol 10-methyl-3-formylphenothiazine (0.037 g), 0.15 mmol 4-chloroacetophenone (0.025 g) and NaOH catalyst was subjected to microwave irradiation. The reaction product (orange powder, m.p. 153 °C) was obtained according to procedure i) 0.04 g reaction product using 1 g silica gel solid support, or according to procedure iii) 0.045 g reaction product using 50 mL ethanol solvent.
NMR (400MHz, CHCl3-d1): δH (ppm): 3.32 (s, 3H), 6.73 (d, 1H), 6.76 (d, 1H), 6.90 (m, 1H), 7.07, (d, 1H), 7.11, (m, 1H), 7.27, (d, 1H), 7.35, (m, 2H), 7.39 (d, 2H), 7.64, (d, 1H), 7.88 (d, 2H). δC (ppm) 35.66; 114.44; 114.09; 119.92; 122.61; 123.99, 128.44; 126.28; 127.27; 127.68; 129.33, 129.11, 131.32; 132.63; 138.45, 143.80, 140.24; 144.75; 147.86; 189.62.
(E)-1-(10-methyl-10H phenothiazin-3-yl)-3-(4-chloro-3-nitrophenyl)-propenone 9d
A mixture of 0.15 mmol 10-methyl-3-formylphenothiazine (0.037 g), 0.15 mmol 4-chloro-3-nitroacetophenone (0.03 g) and NaOH catalyst was subjected to microwave irradiation. The reaction product (dark red powder, m.p.173 °C with decomposition) was obtained according to procedure i) 0.05 g reaction product using 1 g silica gel solid support, or according to procedure iii) 0.06 g reaction product using 50 mL ethanol solvent.
NMR (400MHz, CHCl3-d1): δH (ppm): 3.42 (s, 3H), 6.79 (t, 1H), 6.81 (d, 1H), 6.84 (d, 1H), 7.14 (dd, 1H), 7.19 (m, 1H), 7.31, (d, 1H), 7.44 (m, 2H), 7.69 (d, 1H), 7.78 (d, 1H), 8.14 (dd, 1H), 8.48 (d, 1H). δC (ppm) 35.63, 114.12, 114.55, 117.66, 121.4; 123.30, 125.30, 126.59, 126.24; 127.28, 127.75, 129.66, 130.32; 132.38, 134.76; 136.98; 137.44; 144.26; 145.12; 146.06; 149.34; 189.62.
(E)-1-(10-methyl-10H phenothiazin-3-yl)-3-(4-methoxyphenyl)-propenone 9e
A mixture of 0.15 mmol 10-methyl-3-formylphenothiazine (0.037 g), 0.18 mmol 4-methoxyacetophenone (0.028 g) and NaOH catalyst was subjected to microwave irradiation. The reaction product (yellow powder, m.p. 197 °C) was obtained according to procedure i) 0.044 g reaction product using 1 g silica gel solid support, or according to procedure ii) 0.047 g reaction product using 50 mL ethanol solvent.
NMR (400MHz, CHCl3-d1): δH (ppm): 3.40 (s; 3H), 3.89 (s; 3H), 6.80 (d, 1H), 6.83 (dd; 1H), 6.96 (m, 1H) 6.98 (d, 2H), 7.14 (dd; 1H), 7.18 (m, 1H), 7.40 (dd, 1H), 7.41 (d; 1H) 7.42 (d, 1H), 7.70 (d, 1H), 8.03 (d; 1H). δC (ppm): 35.5; 55.5; 113.8; 114.1; 114.4; 119.7; 122.6; 123.0; 123.9; 126.2; 127.2; 127.7; 129.0; 129.5; 130.7; 131.3; 142.9; 144.8; 147.6; 163.3; 188.5.
(E)-1-(10-methyl-10H phenothiazin-3-yl)-3-(2-naphthyl)-propenone 9f
A mixture of 0.15 mmol 10-methyl-3-formylphenothiazine (0.037 g), 0.17 mmol 2-acetyl-naphthaline (0.03 g) and NaOH catalyst was subjected to microwave irradiation. The reaction product (yellow powder, m.p. 161 °C) was obtained according to procedure i) 0.05 g reaction product using 1 g silica gel solid support, or according to procedure ii) 0.06 g reaction product using 50 mL ethanol solvent.
NMR (400MHz, CHCl3-d1): δH (ppm): 3.41 (s, 3H), 6.82 (d, 1H), 6.84 (d, 1H), 6.97 (m, 1H), 7.15 (d, 1H), 7.19 (m, 1H), 7.45 (dd, 1H), 7.49 (d, 1 H), 7.56 (m, 1H), 7.60 (m, 2H), 7.70 (d, 1H), 7.90 (dd, 1H) 7.93 (dd, 1H), 8.01 (dd, 1H) 8.09 (dd, 1H), 8.53 (dd, 1H). δC (ppm): 35.6, 114.1, 114.4, 119.9, 122.6, 123.1, 124.0, 126.3, 127.3, 127.7, 128.5, 129.8, 143.7, 144.8, 147.9, 190.1, 124.5; 126.7; 129.4 (two overlapping peaks); 127.8, 128.3, 132.6 (two overlapping peaks), 135.4; 135.8
2,6-Bis[3-(10-methyl-10H phenothiazin-3-yl)-1-oxopropenyl]-pyridine 10
A mixture of 2 mmol 10-methyl-3-formylphenothiazine (0.48 g), 1 mmol 2,6 diacetyl-pyridine (0.16 g) and NaOH catalyst was subjected to microwave irradiation. The reaction product (orange powder, m.p. 151 °C) was obtained according to procedure i) 0.30 g reaction product using 2 g silica gel solid support, or according to procedure iii) 0.42 g reaction product using 50 mL ethanol solvent.
NMR (400MHz, CHCl3-d1): δH (ppm): 3.43 (s, 6H), 6.84 (d, 2H), 6.94, (m, 4H), 7.10, (d, 2H), 7.19 (t, 2H), 7.54 (s, 2H), 7.58 (d, 2H), 7.94 (d, 2H), 8.06 (t, 1H), 8.29 (d, 2H), 8.34 (d, 2H). δC (ppm): 35.32; 114.44; 114.09; 119.6; 119.92; 122.61; 123.99, 128.44; 126.28; 127.68; 129.33, 129.11, 132.63; 138.45, 143.80; 144.75; 147.86; 153.6; 187.6.
3,7-Bis[3-(4-nitrophenyl)-1-oxopropenyl]-10-propyl-10H-phenothiazine 11
A mixture of 0.18 mmol 10-propyl-3,7-diacethylphenothiazine (0.06 g), 0.39 mmol 4-nitrobenzaldehyde(0.06 g) and NaOH catalyst was subjected to microwave irradiation. The reaction product (dark red powder, m.p.157 °C) was obtained according to procedure i) 0.07 g reaction product using 1 g silica gel solid support, or according to procedure iii) 0.09 g reaction product using 50 mL ethanol solvent.
NMR (400MHz, CHCl3-d1): δH (ppm): 1.06 (t, 3H), 1.88 (m, 2H), 3.92 (t, 2H), 6.91 (d, 2H), 7.58 (d, 2H), 7.81 (d, 2H), 7.78 (m, 6H), 7.87 (dd, 2H), 8.24, (d, 4H). δC (ppm): 11.8; 20.6; 42.4; 116.2; 119.8; 121.2; 121.4; 127.3,;127.8; 129.2; 131.4; 141.2;145.4; 147.6; 148.2; 189.6.