Threading Cyclodextrins in Chloroform: A [2]Pseudorotaxane
Abstract
:1. Introduction
2. Results and Discussion
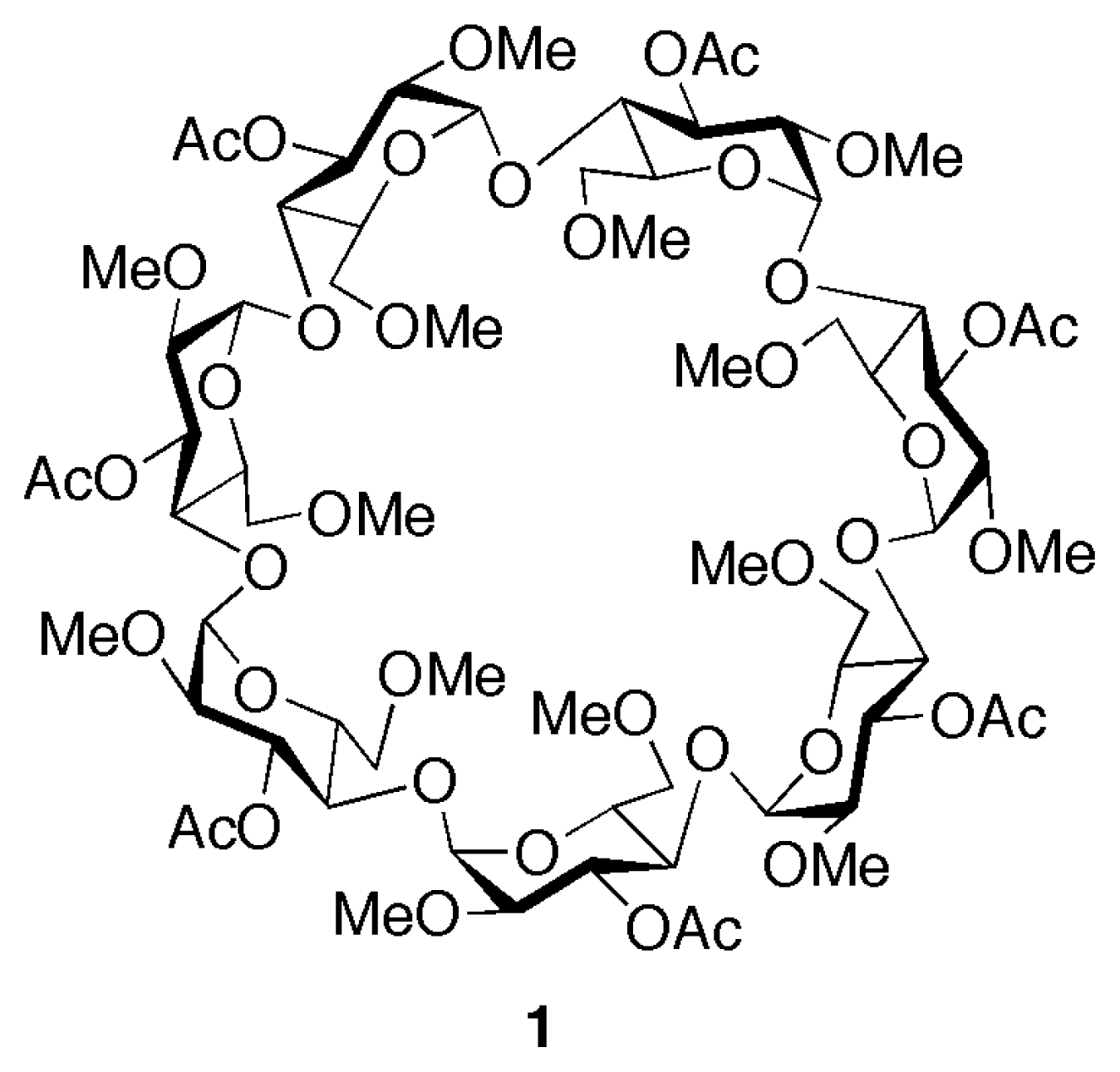
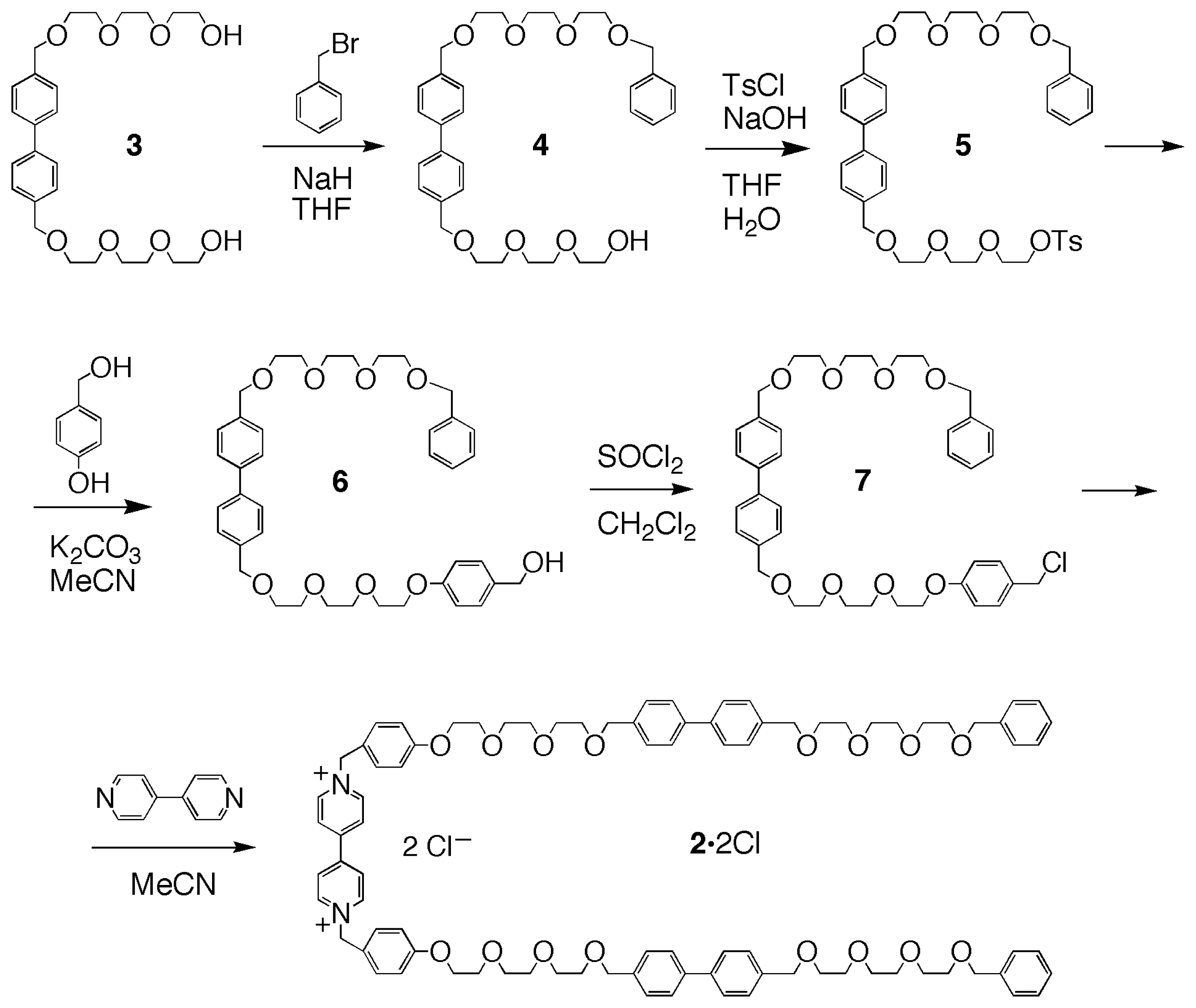
2.1. Design and Synthesis of the Linear and Macrocyclic Components
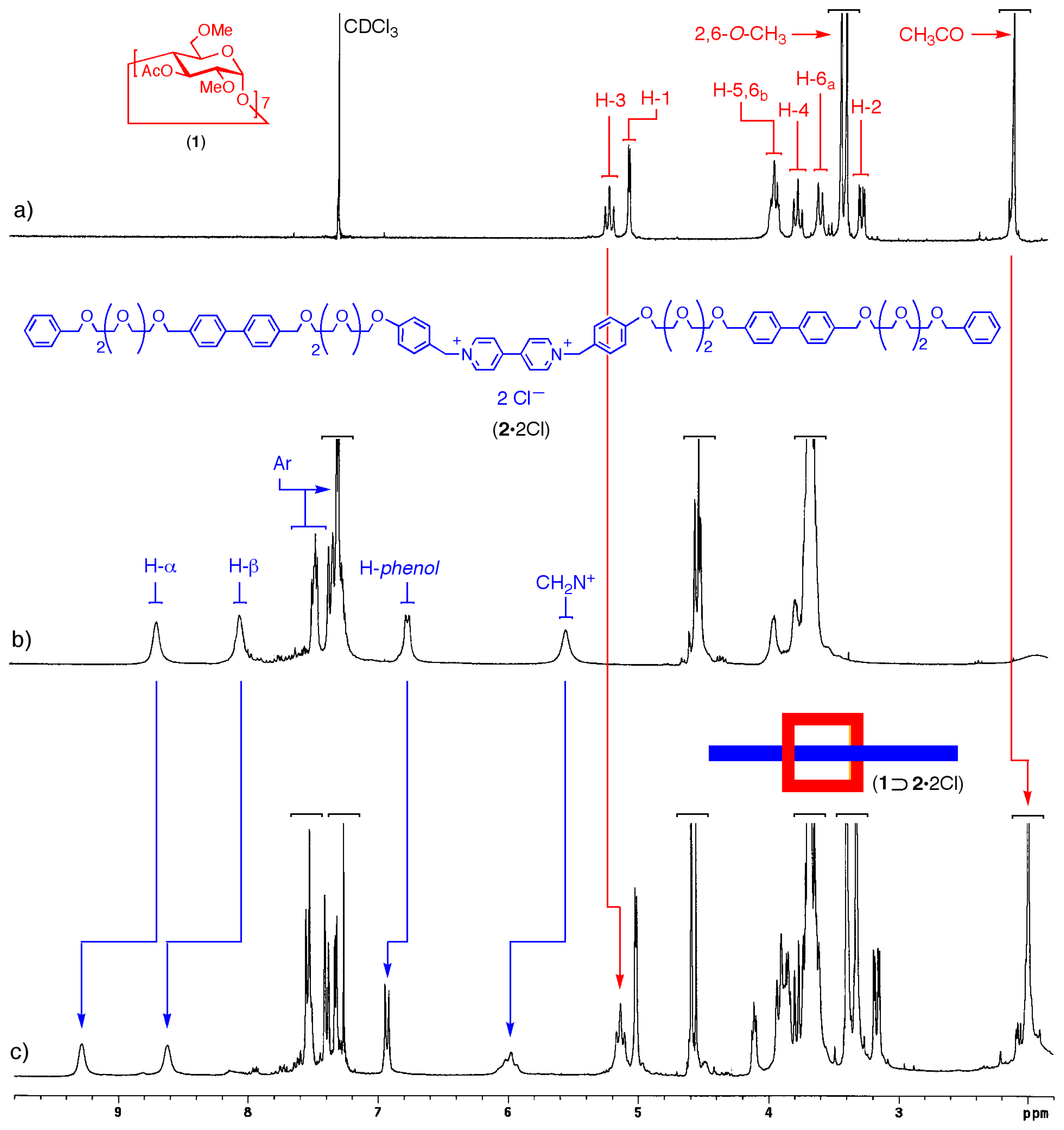
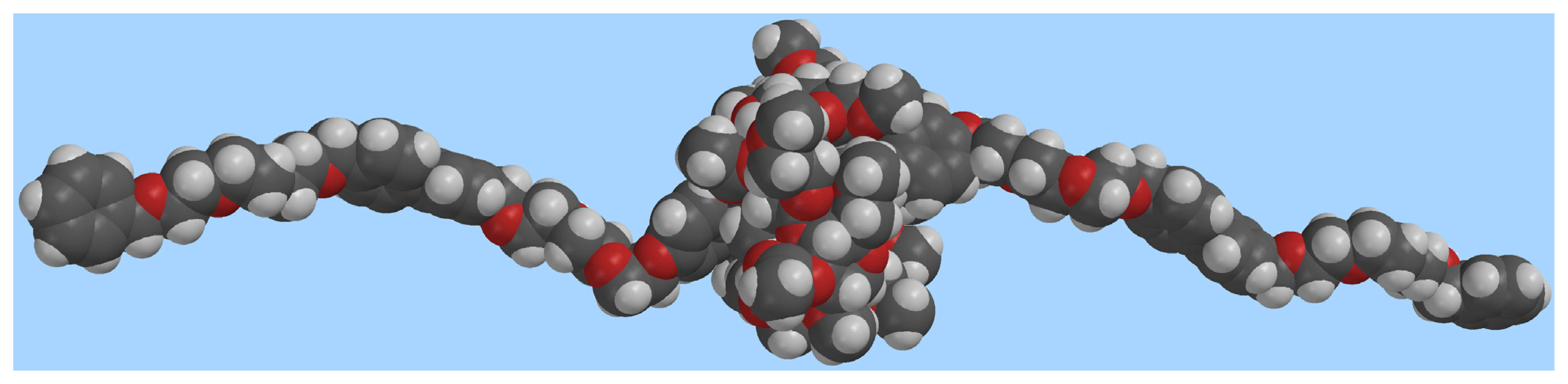
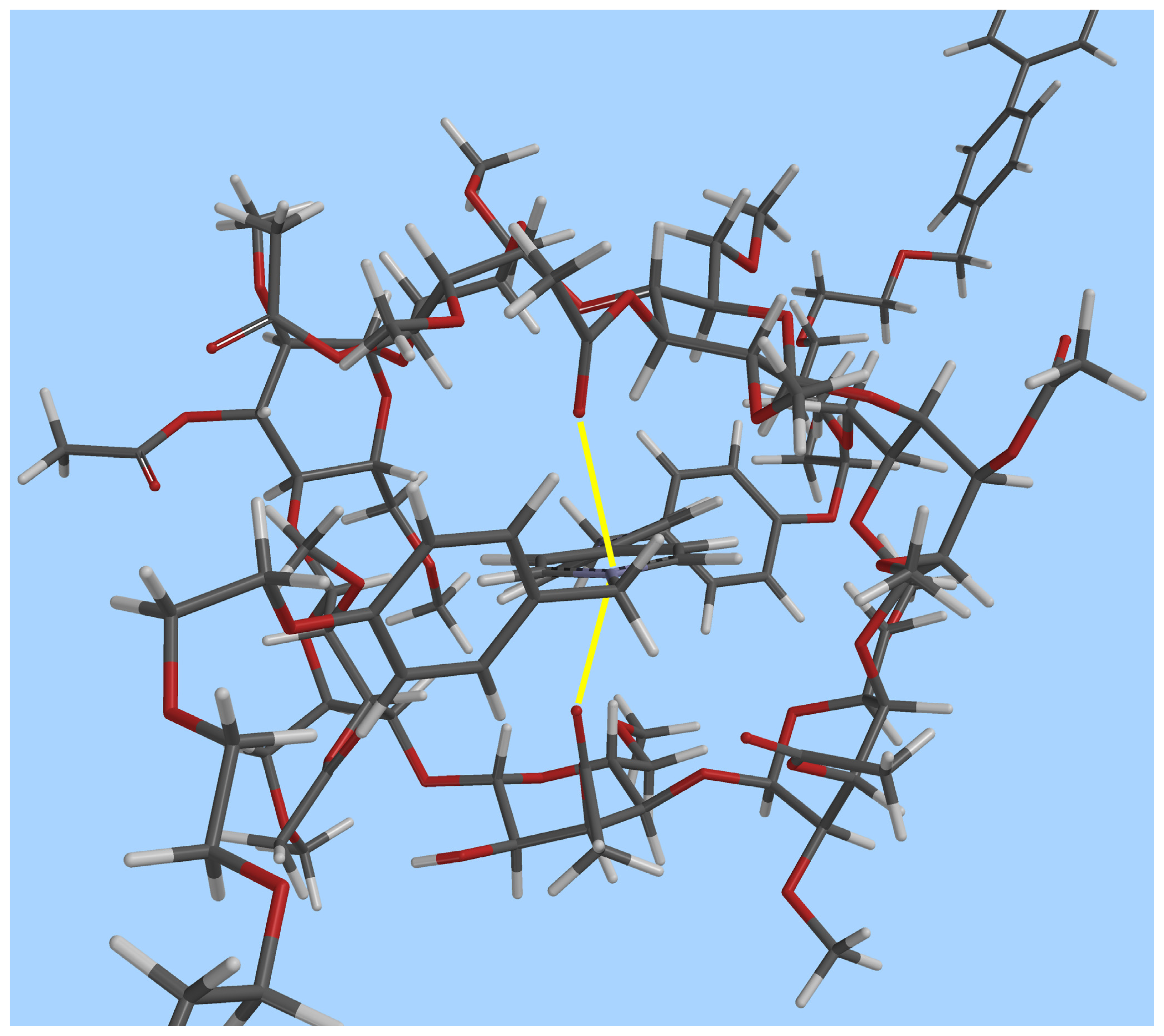
2.2. Self-assembly of the [2]Pseudorotaxane 1⊃2·2Cl
Conclusions
3. Experimental Section
3.1. General Methods
3.2. 1H NMR Binding experiments
3.3. Synthetic Procedures
| Ka in D2O/CD3COCD3 (4:1 v/v) | Ka in CD3COCD3 | |
|---|---|---|
| 1⊃3 | 2900 ± 250 | no binding |
| 1⊃82+ | no bindingA | 13200 ± 1100 B |
Acknowledgements
References and Notes
- Armspach, D.; Gattuso, G.; Koeniger, R.; Stoddart, J. F. Bioorganic Chemistry: Carbohydrates; Hecht, S.M., Ed.; Oxford University Press: New York, 1999; pp. 458–488. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev 1998, 98, 1743–1754. [Google Scholar]
- Comprehensive Supramolecular Chemistry: Cyclodextrins; Szejtli, J.; Osa, T. (Eds.) Elsevier: Oxford, 1996; Volume 3.
- Hedges, A.R. Industrial applications of cyclodextrins. Chem. Rev 1998, 98, 2035–2044. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and their uses: a review. Process Biochem 2004, 39, 1033– 1046. [Google Scholar]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem. Rev 1998, 98, 2045–2076. [Google Scholar]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv. Drug Deliver. Rev 1999, 36, 17–28. [Google Scholar]
- Stoddart, J.F. Cyclodextrins, off-the-shelf components for the construction of mechanically interlocked molecular-systems. Angew. Chem., Int. Ed. Engl 1992, 31, 846–848. [Google Scholar]
- Wenz, G. Cyclodextrins as building-blocks for supramolecular structures and functional units. Angew. Chem., Int. Ed. Engl 1994, 33, 803–822. [Google Scholar]
- Wenz, G.; Han, B.H.; Muller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev 2006, 106, 782–817. [Google Scholar]
- Harada, A. Cyclodextrin-based molecular machines. Acc. Chem. Res 2001, 34, 456–464. [Google Scholar]
- Nepogodiev, S.A.; Stoddart, J.F. Cyclodextrin-based catenanes and rotaxanes. Chem. Rev 1998, 98, 1959–1976. [Google Scholar]
- Onagi, H.; Blake, C.J.; Easton, C.J.; Lincoln, S.F. Installation of a ratchet tooth and pawl to restrict rotation in a cyclodextrin rotaxane. Chem. Eur. J 2003, 9, 5978–5988. [Google Scholar]
- Stanier, C.A.; Alderman, S.J.; Claridge, T.D.W.; Anderson, H.L. Unidirectional photoinduced shuttling in a rotaxane with a symmetric stilbene dumbbell. Angew. Chem. Int. Ed 2002, 41, 1769–1772. [Google Scholar]
- Armspach, D.; Ashton, P.R.; Ballardini, R.; Balzani, V.; Godi, A.; Moore, C.P.; Prodi, L.; Spencer, N.; Stoddart, J.F.; Tolley, M.S.; Wear, T.J.; Williams, D.J. Catenated cyclodextrins. Chem. Eur. J 1995, 1, 33–55. [Google Scholar]
- Harada, A.; Hashidzume, A.; Takashima, Y. Cyclodextrin-based supramolecular polymers. Polym. Sci 2006, 201, 1–43. [Google Scholar]
- Suh, J. Synthetic artificial peptidases and nucleases using macromolecular catalytic systems. Acc. Chem. Res 2003, 36, 562–570. [Google Scholar]
- Breslow, R.; Dong, S.D. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev 1998, 98, 1997–2012. [Google Scholar]
- Saha, S.; Stoddart, J.F. Photo-driven molecular devices. Chem. Soc. Rev 2007, 36, 77–92. [Google Scholar]
- Tian, H.; Wang, Q.C. Recent progress on switchable rotaxanes. Chem. Soc. Rev 2006, 35, 361– 374. [Google Scholar]
- Mirzoian, A.; Kaifer, A.E. Reactive pseudorotaxanes: Inclusion complexation of reduced viologens by the hosts β-cyclodextrin and heptakis(2,6-di-O-methyl)-β-cyclodextrin. Chem. Eur. J 1997, 3, 1052–1058. [Google Scholar]
- Murakami, M.; Kawabuchi, A.; Matsumoto, R.; Ido, T.; Nakashima, N. A multi-mode-driven molecular shuttle: photochemically and thermally reactive azobenzene rotaxanes. J. Am. Chem. Soc 2005, 127, 15891–15899. [Google Scholar]
- Murakami, M.; Kawabuchi, A.; Kotoo, K.; Kunitake, M.; Nakashima, N. A light-driven molecular shuttle based on a rotaxane. J. Am. Chem. Soc 1997, 119, 7605–7606. [Google Scholar]
- Ma, X.; Wang, Q.C.; Qu, D.H.; Xu, Y.; Ji, F.Y.; Tian, H. A light-driven pseudo[4]rotaxane encoded by induced circular dichroism in a hydrogel. Adv. Funct. Mater 2007, 17, 829–837. [Google Scholar]
- Liu, Y.; Li, X.-Y.; Zhang, H.-Y.; Li, C.-J.; Ding, F. Cyclodextrin-driven movement of cucurbit[7]uril. J. Org. Chem 2007, 72, 3640–3645. [Google Scholar]
- Saenger, W. Inclusion Compounds; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Eds.; Academic Press: London, 1984; Volume 2, pp. 231–259. [Google Scholar]
- Engeldinger, E.; Armspach, D.; Matt, D. Capped cyclodextrins. Chem. Rev 2003, 103, 4147– 4173. [Google Scholar]
- Rauf Khan, A.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev 1998, 98, 1977–1996. [Google Scholar]
- Ashton, P.R.; Boyd, S. E.; Gattuso, G.; Hartwell, E. Y.; Koeniger, R.; Spencer, N.; Stoddart, J. F. A novel approach to the synthesis of some chemically-modified cyclodextrins. J. Org. Chem 1995, 60, 3898–3903. [Google Scholar]
- Morozumi, T.; Sato, N.; Nakamura, H. Selective pseudo-rotaxane type complex formation of zinc(II) (t-butylatedtetraphenyl) porphyrin-viologen linked compounds with tri-O-methyl-β-cyclodextrin. J. Incl. Phenom. Macro 2006, 56, 141–148. [Google Scholar]
- Wenz, G.; Wolf, F.; Wagner, M.; Kubik, S. Topology and molecular chemistry – synthesis, topography and stability of a [2]-rotaxane derived from a lipophilic cyclodextrin derivative. New J. Chem 1993, 17, 729–738. [Google Scholar]
- Wenz, G.; Vonderbey, E.; Schmidt, L. Synthesis of a lipophilic cyclodextrin-[2]-rotaxane. Angew. Chem., Int. Ed. Engl 1992, 31, 783–785. [Google Scholar]
- Sliwa, W.; Bachowska, B. Supramolecular species bearing quaternary azaaromatic moieties. Heterocycles 2006, 68, 1467–1500. [Google Scholar]
- Cafeo, G.; Gargiulli, C.; Gattuso, G.; Kohnke, F.H.; Notti, A.; Occhipinti, S.; Pappalardo, S.; Parisi, M.F. Recognition and binding of paraquat dichloride by cyclodextrin/calix[6]pyrrole binary host systems. Tetrahedron Lett 2002, 43, 8103–8106. [Google Scholar]
- Cafeo, G.; Gattuso, G.; Kohnke, F.H.; Notti, A.; Occhipinti, S.; Pappalardo, S.; Parisi, M.F. Remarkable boosting of the binding of ion-paired organic salts by binary host systems. Angew. Chem. Int. Ed 2002, 41, 2122–2126. [Google Scholar]
- Giastas, P.; Mourtzis, N.; Yannakopoulou, K.; Mavridis, I.M. Pseudorotaxanes of β-cyclodextrin with diamino end-functionalized oligo-phenyl and -benzyl compounds in solution and in the solid state. J. Incl. Phenom. Macro 2002, 44, 247–250. [Google Scholar]
- Park, J.W.; Song, H.J.; Chang, H.J. Unidirectional α-cyclodextrin-based [2]rotaxanes bearing viologen unit on axle. Tetrahedron Lett 2006, 47, 3831–3834. [Google Scholar]
- Oshikiri, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. Kinetic control of threading of cyclodextrins onto axle molecules. J. Am. Chem. Soc 2005, 127, 12186–12187. [Google Scholar]
- Park, J.W.; Song, H.J. Isomeric [2]rotaxanes and unidirectional [2]pseudorotaxane composed of α-cyclodextrin and aliphatic chain-linked carbazole-viologen compounds. Org. Lett 2004, 6, 4869– 4872. [Google Scholar]
- Baer, A.J.; Macartney, D.H. Orientational isomers of α-cyclodextrin [2]semi-rotaxanes with asymmetric dicationic threads. Org. Biomol. Chem 2005, 3, 1448–1452. [Google Scholar]
- Park, J.W.; Song, H.J.; Lee, S.Y. Face selectivity of inclusion complexation of viologens with β-cyclodextrin and 6-O-(2-sulfonato-6-naphthyl)-β-cyclodextrin. J. Phys. Chem. B 2002, 106, 7186–7192. [Google Scholar]
- Cyclodextrin 1 was prepared according to a literature procedure and its spectroscopic data were in full agreement with those previously published. See: Hirayama, F.; Mieda, S.; Miyamoto, Y.; Arima, H.; Uekama, K. Heptakis(2,6-di-O-methyl-3-O-acetyl)-β-cyclodextrin: a water-soluble cyclodextrin derivative with low hemolytic activity. J. Pharm. Sci 1999, 88, 970–975. [Google Scholar]
- Alston, D.R.; Lilley, T.H.; Stoddart, J.F. The binding of cyclobutane-1,1- dicarboxylatodiamineplatinum(II) by α-cyclodextrin in aqueous solution. J. Chem. Soc. Chem. Commun 1985, 1600–1602. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals; Pergamon Press: Oxford, 1989. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Gattuso, G.; Gargiulli, C.; Parisi, M.F. Threading Cyclodextrins in Chloroform: A [2]Pseudorotaxane. Int. J. Mol. Sci. 2007, 8, 1052-1063. https://doi.org/10.3390/i8101052
Gattuso G, Gargiulli C, Parisi MF. Threading Cyclodextrins in Chloroform: A [2]Pseudorotaxane. International Journal of Molecular Sciences. 2007; 8(10):1052-1063. https://doi.org/10.3390/i8101052
Chicago/Turabian StyleGattuso, Giuseppe, Claudia Gargiulli, and Melchiorre F. Parisi. 2007. "Threading Cyclodextrins in Chloroform: A [2]Pseudorotaxane" International Journal of Molecular Sciences 8, no. 10: 1052-1063. https://doi.org/10.3390/i8101052




