Effect of Turmeric, Turmerin and Curcumin on Ca2+, Na/K+ Atpases in Concanavalin A-Stimulated Human Blood Mononuclear Cells
Abstract
:Introduction
Materials and Methods
Chemicals
Cells
Determination of Na/K+ATPases Activity
Determination of Ca2+ATPase Activity
Protein Assay
Statistical Analysis
Results
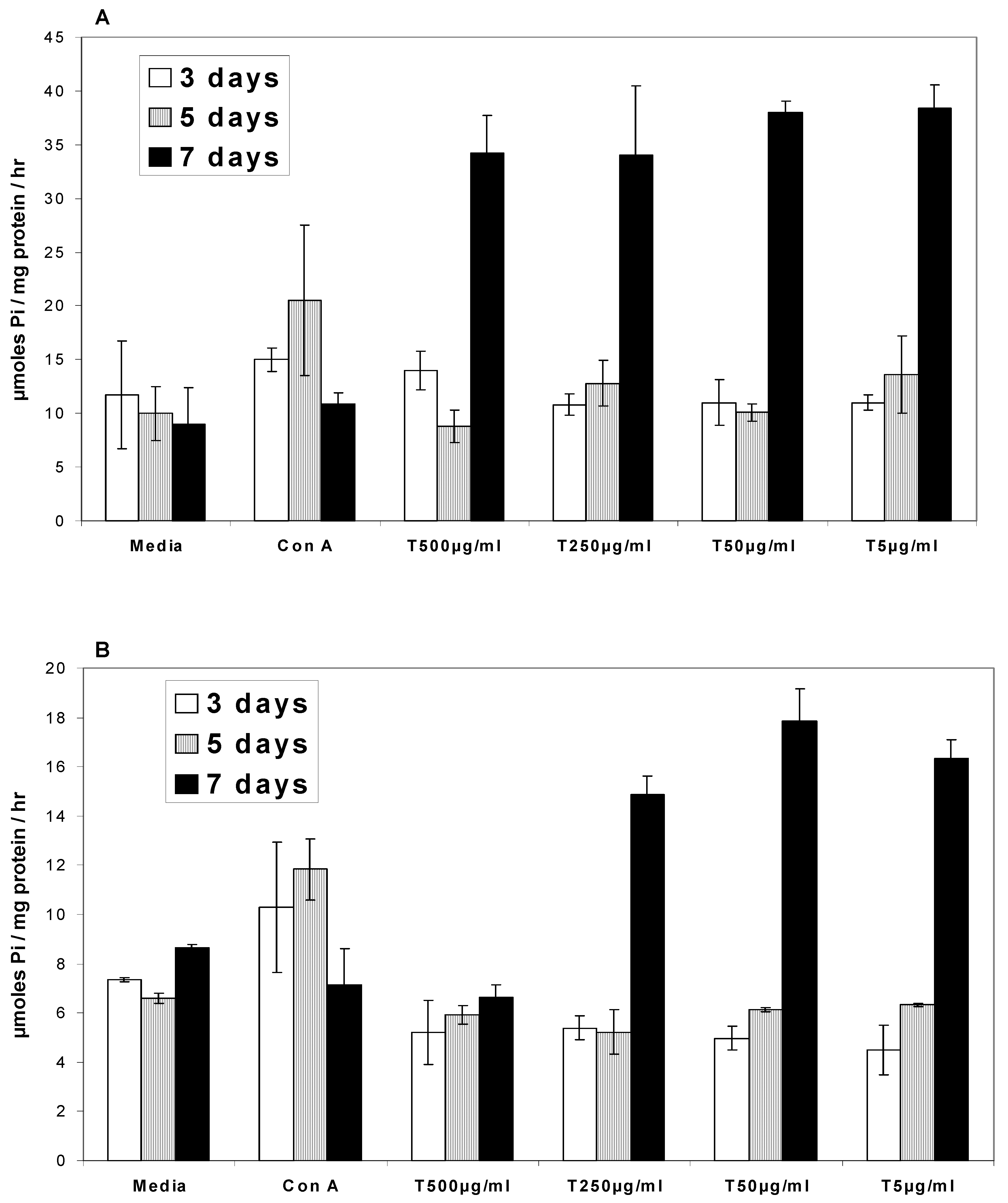
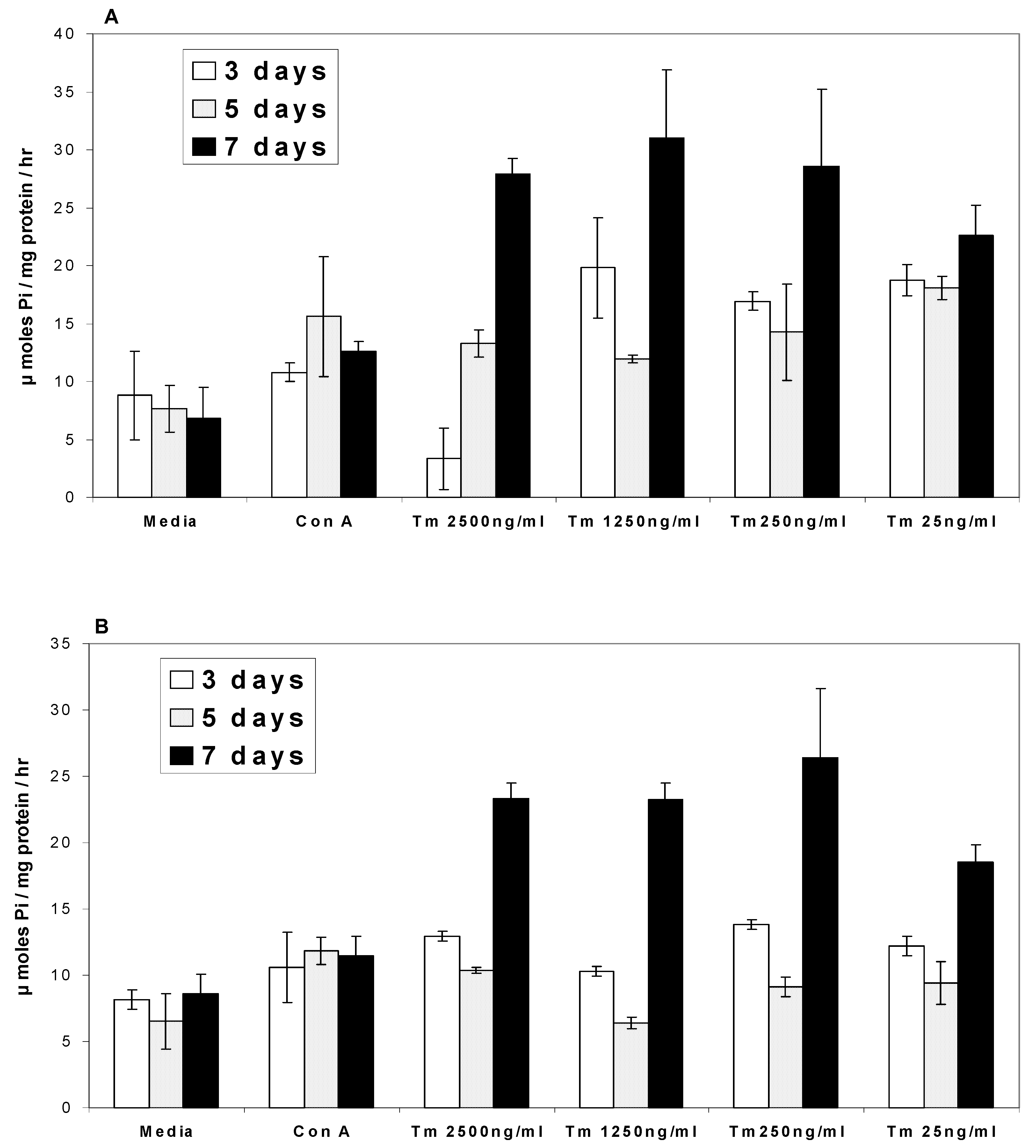
Ca2+ATPases
Na/K+ATPases

Discussion
- 1)
- In the presence of turmeric, Ca2+ATPase and Na/K+ATPase levels generally decreased above the base level at 3 and 5 days while at 7 days the ATPase activity level exhibited a two-fold increase.
- 2)
- In the presence of curcumin, Ca2+ATPase and Na/K+ATPase levels decreased below the base level at 3 and 5 days while at 7 days ATPase activity exhibited a two-fold increase.
- 3)
- In the presence of turmerin, Ca2+ATPase and Na/K+ATPase levels generally increased on day 3, decreased on day 5 and increased two-fold on day 7.
Acknowledgements
References
- Papp, B.; Enyedi, A.; Paszty, K.; Kovacs, T.; Sarkadi, B.; Gardos, G.; Magnier, C.; Wuytack, F.; Enouf, J. Simultaneous presence of two distinct endoplasmic-reticulum-type calcium-pump isoforms in human cells. Biochem. J. 1992, 288, 297–302. [Google Scholar] [PubMed]
- Schwartz, A.; Lindenmayer, G. E.; Allen, J. C. The sodium-potassium adenosine triphosphatase: Physiological, pharmacological and biochemical aspects. Pharmacol. Rev. 1975, 27, 3–134. [Google Scholar] [PubMed]
- Thomas, R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol. Rev. 1972, 52, 563–594. [Google Scholar] [PubMed]
- Crane, R. K. The gradient hypothesis and other models of carrier mediated active transport. Rev. Physiol. Biochem. Pharmacol. 1977, 69–159. [Google Scholar]
- Iversen, L. L.; Kelly, J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem. Pharmacol. 1975, 24, 933–938. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, C.; Saida, K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu. Rev. Physiol. 1989, 51, 315–329. [Google Scholar]
- Min, W.; Dunn, A. J.; Jones, D. H. Non-glycosylated recombinant pro-concanavalin A is active without polypeptide cleavage. EMBO J. 1992, 11, 1303–1307. [Google Scholar] [PubMed]
- Ruscetti, F. W.; Chervenick, P. A. Regulation of the release of colony-stimulating activity from mitogen-stimulated lymphocytes. J. Immunol. 1975, 114, 1513–1517. [Google Scholar] [PubMed]
- Novogrodsky, A.; Katchalski, E. Lymphocyte Transformation Induced by Concanavalin A and its Reversion by Methyl-[[alpha]]-Mannopyranoside. Biochim. Biophys. Acta. 1971, 228, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Perlmann, P.; Nilsson, H.; Leon, M. Inhibition of Cytotoxicity of Lymphocytes by Concanavalin A in-vitro. Science 1970, 168, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kobayashi, T. Effects of Concanavalin A on Cytokine mRNA Expression in Mouse Liver. J. Pharmacol. 1997, 75, 199–201. [Google Scholar]
- Wermerskirchen, A. S.; LaTocha, D. H.; Clarke, B. L. Adrenocorticotropic hormone controls Concanavalin A activation of rat lymphocytes by modulating IL-2 production. Life Sci. 2000, 67, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Arai, K. I.; Lee, F.; Miyajima, A.; Miyatake, S.; Arai, N.; Yokota, T. Cytokines: coordinators of immune and inflammatory responses. Annu. Rev. Biochem. 1990, 59, 783–836. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, L.; Shalini, V. K.; Shylaja, M. Turmerin: a water soluble antioxidant peptide from turmeric [Curcuma longa]. Arch. Biochem. Biophys. 1992, 292, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Selvam, R.; Subramanian, L.; Gayathri, R.; Angayarkanni, N. The anti-oxidant activity of turmeric (Curcuma longa). J. Ethnopharmacol. 1995, 47, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Nakagawa, K.; Miyazawa, T. Antioxidative effects of turmeric, rosemary and capsicum extracts on membrane phospholipid peroxidation and liver lipid metabolism in mice. Biosci. Biotechnol. Biochem. 1999, 63, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Frautschy, S. A.; Hu, W.; Kim, P.; Miller, S. A.; Chu, T.; Harris-White, M. E.; Cole, G. M. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging 2001, 22, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Phan, T. T.; See, P.; Lee, S. T.; Chan, S. Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J. Trauma 2001, 51, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Krishnakanth, T. P. Effect of retinol deficiency and curcumin or turmeric feeding on brain Na(+)-K+ adenosine triphosphatase activity. Mol. Cell Biochem. 1994, 137, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. C.; Jan, T. R.; Yeh, S. F. Inhibitory effect of curcumin, an anti-inflammatory agent, on vascular smooth muscle cell proliferation. Eur. J. Pharmacol. 1992, 221, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Fritz, F. P.; Hamrich, M. E. Enzymatic analysis of ATPase. Enzymol. Acta. Biocat. 1966, 30, 57–64. [Google Scholar]
- Desaiah, D.; Ho, I. K. Effects of acute and continuous morphine administration of catelcholamine sensitive ATPase in mouse brains. J. Pharmacol. Exp. Ther. 1979, 208, 80–85. [Google Scholar] [PubMed]
- Fiske, C. H.; Subba Row, Y. The colorimetric determination of phosphorous. J. Biol. Chem. 1921, 66, 375–400. [Google Scholar]
- Wright, P.; Quastel, M. R.; Kaplan, J. G. Differential sensitivity of antigen- and mitogen-stimulated human leukocytes to prolonged inhibition of potassium transport. Exp. Cell Res. 1973, 79, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J. L.; Hokin, L. E. The sodium-potassium adenosinetriphosphatase. Annu. Rev. Biochem. 1974, 43, 327–356. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A. H.; Segel, G. B.; Lichtman, M. A. Effect of Trifluperazine and mitogenic lectins on calcium ATPase activity and calcium transport by human lymphocyte plasma membrane vesicles. J. Cell. Physiol. 1982, 111, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bielak-Zmijewska, A.; Piwocka, K.; Skierski, J.; Radziszewska, E. Inhibition of proliferation and apoptosis of human and rat T lymphocytes by curcumin, a curry pigment. Biochem. Pharmacol. 1997, 54, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Yasni, S.; Yoshiie, K.; Oda, H.; Sugano, M.; Imaizumi, K. Dietary Curcuma xanthorrhiza Roxb. increases mitogenic responses of splenic lymphocytes in rats, and alters populations of the lymphocytes in mice. J. Nutr. Sci. Vitaminol. (Tokyo) 1993, 39, 345–354. [Google Scholar] [CrossRef]
- Rossier, B. C.; Geering, K.; Kraenbuhl, J. P. Regulation of the sodium pump: how and why? TIBS 1987, 2, 483–487. [Google Scholar]
- Logan-Smith, M. J.; East, J. M.; Lee, A. G. Evidence for a global inhibitor-induced conformation change on the Ca(2+)-ATPase of sarcoplasmic reticulum from paired inhibitor studies. Biochemistry 2002, 41, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Bilmen, J. G.; Khan, S. Z.; Javed, M. H.; Michelangeli, F. Inhibition of the SERCA Ca2+ pumps by curcumin. Curcumin putatively stabilizes the interaction between the nucleotide-binding and phosphorylation domains in the absence of ATP. Eur. J. Biochem. 2001, 268, 6318–6327. [Google Scholar] [CrossRef] [PubMed]
- Sumbilla, C.; Lewis, D.; Hammerschmidt, T.; Inesi, G. The slippage of the Ca2+ pump and its control by anions and curcumin in skeletal and cardiac sarcoplasmic reticulum. J. Biol. Chem. 2002, 277, 13900–13906. [Google Scholar] [CrossRef] [PubMed]
- Sarmay, G.; Istvan, L.; Gergely, J. Shedding and reappearance of Fc, C3 and SRBC receptors on peripheral lymphocytes from normal donors and chronic lymphatic leukaemia (CLL) patients. Immunology 1978, 34, 315–321. [Google Scholar] [PubMed]
- Romagnani, S.; Maggi, E.; Lorenzini, M.; Giudizi, G. M.; Biagiotti, R.; Ricci, M. Study of some properties of the receptor for IgM on human lymphocytes. Clin. Exp. Immunol. 1979, 36, 502–510. [Google Scholar] [PubMed]
- McKimm-Breschkin, J. L.; Miller, J. F. Synthesis and turnover of the putative T-lymphocyte antigen receptor. Scand. J. Immunol. 1985, 21, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Dello, S. P.; Rovida, E. Transmodulation of cell surface regulatory molecules via ectodomain shedding. Biol. Chem. 2002, 383, 69–83. [Google Scholar] [PubMed]
© 2003 by MDPI (http://www.mdpi.org).
Share and Cite
Cohly, H.H.P.; Rao, M.-R.; Kanji, V.K.; Patlolla, B.; Taylor, A.; Wilson, M.T.; Angel, M.F.; Das, S.K. Effect of Turmeric, Turmerin and Curcumin on Ca2+, Na/K+ Atpases in Concanavalin A-Stimulated Human Blood Mononuclear Cells. Int. J. Mol. Sci. 2003, 4, 34-44. https://doi.org/10.3390/i4020034
Cohly HHP, Rao M-R, Kanji VK, Patlolla B, Taylor A, Wilson MT, Angel MF, Das SK. Effect of Turmeric, Turmerin and Curcumin on Ca2+, Na/K+ Atpases in Concanavalin A-Stimulated Human Blood Mononuclear Cells. International Journal of Molecular Sciences. 2003; 4(2):34-44. https://doi.org/10.3390/i4020034
Chicago/Turabian StyleCohly, Hari H. P., Maheshwara-Rajeswara Rao, Vijaya K. Kanji, Babu Patlolla, Anelle Taylor, Melanie T. Wilson, Michael F. Angel, and Suman K. Das. 2003. "Effect of Turmeric, Turmerin and Curcumin on Ca2+, Na/K+ Atpases in Concanavalin A-Stimulated Human Blood Mononuclear Cells" International Journal of Molecular Sciences 4, no. 2: 34-44. https://doi.org/10.3390/i4020034



