Energy-Dependent Endocytosis Is Responsible for Skin Penetration of Formulations Based on a Combination of Indomethacin Nanoparticles and l-Menthol in Rat and Göttingen Minipig
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Physical Properties in Transdermal Formulation Based on a Combination of IND-NPs and l-Menthol
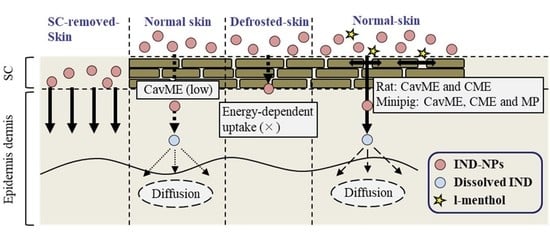
2.2. Changes in Transdermal Penetration of IND-NPs in the Normal, SC-Removed, and Defrosted Skin
2.3. Effects of Energy-Dependent Endocytosis on Drug Skin Penetration of the Transdermal Formulation Based on A Combination of IND-NPs and l-Menthol
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Design of the Transdermal Formulation Based on IND-NPs
4.4. Characteristics of Transdermal Formulations Based on IND-NPs
4.5. Measurement of IND by HPLC Method
4.6. In Vitro Skin Penetration of Transdermal Formulation Based on IND-NPs
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.H. Device-assisted transdermal drug delivery. Adv. Drug. Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef]
- Gavin, P.D.; El-Tamimy, M.; Keah, H.H.; Boyd, B.J. Tocopheryl phosphate mixture (TPM) as a novel lipid-based transdermal drug delivery carrier: Formulation and evaluation. Drug Deliv. Transl. Res. 2017, 7, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Gupta, V.; Anselmo, A.C.; Muraski, J.A.; Mitragotri, S. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J. Control. Release 2014, 173, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. Int. J. Pharm. Sci. Res. 2016, 7, 2274–2290. [Google Scholar] [CrossRef]
- Brambilla, D.; Luciani, P.; Leroux, J.C. Breakthrough discoveries in drug delivery technologies: The next 30 years. J. Control. Release 2014, 190, 9–14. [Google Scholar] [CrossRef]
- Han, T.; Das, D.B. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: A review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug. Dev. Ind. Pharm. 2014, 40, 433–440. [Google Scholar] [CrossRef]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M.; Ali, A.A. Transdermal drug delivery: The inherent challenges and technological advancements. Asian J. Pharm. Sci. 2010, 5, 276–288. [Google Scholar]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int. J. Nanomed. 2018, 13, 5215–5229. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ogata, F.; Ishii, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2138. [Google Scholar] [CrossRef] [Green Version]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Yamaguchi, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Combination with l-Menthol Enhances Transdermal Penetration of Indomethacin Solid Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3644. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshioka, C.; Ito, Y. Pharmacokinetics and Antiinflammatory Effect of a Novel Gel System Containing Ketoprofen Solid Nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.; Kang, H.S.; Park, W.-S.; Han, S.H.; Kim, J.; Chang, I.S. Transdermal delivery of mixnoxidil with block copolymer nanoparticles. J. Control. Release 2004, 97, 477–484. [Google Scholar] [CrossRef]
- Nagai, N.; Iwai, Y.; Sakamoto, A.; Otake, H.; Oaku, Y.; Abe, A.; Nagahama, T. Drug Delivery System Based On Minoxidil Nanoparticles Promotes Hair Growth In C57BL/6 Mice. Int. J. Nanomed. 2019, 14, 7921–7931. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold atmospheric plasma induces ATP-dependent endocytosis of nanoparticles and synergistic U373MG cancer cell death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef] [Green Version]
- Proulx, S.T.; Luciani, P.; Dieterich, L.C.; Karaman, S.; Leroux, J.C.; Detmar, M. Expansion of the lymphatic vasculature in cancer and inflammation: New opportunities for in vivo imaging and drug delivery. J. Control. Release 2013, 172, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, W.; Li, J.; Tao, L.; Wei, Y. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol. Res. 2012, 1, 62–68. [Google Scholar] [CrossRef]
- Youm, I.; Bazzil, J.D.; Otto, J.W.; Caruso, A.N.; Murowchick, J.B.; Youan, B.B. Influence of surface chemistry on cytotoxicity and cellular uptake of nanocapsules in breast cancer and phagocytic cells. AAPS J. 2014, 16, 550–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Zhang, F.; Lu, X.; Wei, X.; Wang, J.; Fang, X.; Si, D.; Wang, Y.; Zhang, C.; Yang, R.; et al. Polymeric micelles for enhanced lymphatic drug delivery to treat metastatic tumors. J. Control. Release 2013, 171, 133–142. [Google Scholar] [CrossRef]
- Rappoport, J.Z. Focusing on clathrin-mediated endocytosis. Biochem. J. 2008, 412, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. More effective nanomedicines through particle design. Small 2011, 7, 1919–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef] [Green Version]
- Simon, G.A.; Maibach, H.I. The pig as an experimental animal model of percutaneous permeation in man: Qualitative and quantitative observations—An overview. Skin Pharmacol. Appl. Skin Physiol. 2000, 13, 229–234. [Google Scholar] [CrossRef]
- Mortensen, J.T.; Brinck, P.; Lichtenberg, J. The minipig in dermal toxicology: A literature review. Scand. J. Lab. Anim. Sci. 1998, 25 (Suppl. 1), 77–83. [Google Scholar]
- Schmook, F.P.; Meingassner, J.G.; Billich, A. Comparison of human skin or epidermis models with human and animal skin in in vitro percutaneous absorption. Int. J. Pharm. 2001, 215, 51–56. [Google Scholar] [CrossRef]
- Weinstein, G.D.; Van Scott, E.J. Autoradiographic analysis of turnover time and protein synthesis in pig epidermis. J. Invest. Dermatol. 1965, 45, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Kaplun-Frischo, Y.; Touitou, E. Testosterone skin permeation enhancement by menthol through formation of eutectic with drug and interaction with skin lipids. J. Pharm. Sci. 1997, 86, 1394–1399. [Google Scholar] [CrossRef]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ying, L.; Wylie, S.; Lee, T.R.; Kim, H.S.; Man, H.; Ho, D.; Decuzzi, P.; Liu, W.K. Multiscale modeling and uncertainty quantification in nanoparticle-mediated drug/gene delivery. Comput. Mech. 2014, 53, 511–537. [Google Scholar] [CrossRef]
- Majumder, P.; Baxa, U.; Walsh, S.T.R.; Schneider, J.P. Design of a multicompartment hydrogel that facilitates time-resolved delivery of combination therapy and Synergized killing of glioblastoma. Angew. Chem. Int. Ed. Engl. 2018, 57, 15040–15044. [Google Scholar] [CrossRef]
- Medina-Kauwe, L.K. “Alternative” endocytic mechanisms exploited by pathogens: New avenues for therapeutic delivery? Adv. Drug Del. Rev. 2007, 59, 798–809. [Google Scholar] [CrossRef] [Green Version]
- Benmerah, A.; Lamaze, C. Clathrin-coated Pits: Vive la différence? Traffic 2007, 8, 970–982. [Google Scholar] [CrossRef]
- Tamaru, M.; Akita, H.; Fujiwara, T.; Kajimoto, K.; Harashima, H. Leptin-derived peptide; a targeting ligand for mouse brain-derived endothelial cells via macropinocytosis. Biochem. Biophys. Res. Commun. 2010, 394, 587–592. [Google Scholar] [CrossRef]
- Swanson, J.A.; Watts, C. Macropinocytosis. Trends. Cell Biol. 1995, 5, 424–428. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Roxbury, D.; Gong, X.; Mukhopadhyay, D.; Jagota, A. DNA conjugated SWCNTs enter endothelial cells via Rac1 mediated macropinocytosis. Nano. Lett. 2012, 12, 1826–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, J.A. Shaping Cups into Phagosomes and Macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [Green Version]





| Formulation | Particle Size nm (Range) | Number of NPs × 1010 Particles/0.3 g | Zeta Potentials mV | Viscosity Pa∙s | Solubility µM |
|---|---|---|---|---|---|
| N-IND | 103.1 (81–193) | 22.6 ± 1.2 | −21.1 ± 0.7 | 5.0 ± 0.1 | 391.2 ± 10.3 |
| N-IND/MEN | 106.3 (79–216) | 22.1 ± 1.4 | −19.9 ± 0.6 | 5.0 ± 0.1 | 408.5 ± 11.7 |
| Treatment | Jc (nmol/cm2/h) | Kp (×10−4 cm/h) | Km (×10−2) | τ (h) | D (×10−4 cm2/h) |
|---|---|---|---|---|---|
| N-IND Control | 43.6 ± 4.3 | 1.55 ± 0.19 | 2.76 ± 0.30 | 2.09 ± 0.08 | 4.04 ± 0.11 |
| Nystatin | 34.9 ± 3.2 * | 1.23 ± 0.15 * | 2.09 ± 0.18 * | 1.90 ± 0.05 | 4.43 ± 0.10 * |
| Dynasore | 43.2 ± 4.8 | 1.54 ± 0.23 | 2.72 ± 0.35 | 2.08 ± 0.09 | 4.07 ± 0.13 |
| Rottlerin | 43.7 ± 4.1 | 1.52 ± 0.18 | 2.69 ± 0.29 | 2.08 ± 0.08 | 4.09 ± 0.11 |
| N-IND/MEN Control | 158.7 ± 5.7 | 5.69 ± 0.19 | 11.0 ± 0.33 | 2.31 ± 0.04 | 3.78 ± 0.13 |
| Nystatin | 53.6 ± 0.38 # | 1.69 ± 0.18 # | 3.15 ± 0.29 # | 2.10 ± 0.07 # | 4.02 ± 0.12 |
| Dynasore | 61.2 ± 0.43 # | 1.78 ± 0.19 # | 3.86 ± 0.38 # | 2.13 ± 0.05 # | 3.95 ± 0.13 |
| Rottlerin | 159.1 ± 5.9 | 5.71 ± 0.20 | 11.3 ± 0.31 | 2.30 ± 0.04 | 3.80 ± 0.15 |
| Treatment | Jc (nmol/cm2/h) | Kp (×10−4 cm/h) | Km (×10−2) | τ (h) | D (×10−4 cm2/h) |
|---|---|---|---|---|---|
| N-IND Control | 11.7 ± 1.14 | 4.16 ± 0.39 | 5.10 ± 0.50 | 1.46 ± 0.08 | 5.78 ± 0.33 |
| Nystatin | 8.44 ± 0.41 | 3.03 ± 0.13 * | 3.48 ± 0.06 * | 1.36 ± 0.09 | 6.15 ± 0.39 |
| Dynasore | 12.9 ± 0.31 | 4.59 ± 0.11 | 4.57 ± 0.34 | 1.20 ± 0.06 | 7.09 ± 0.41 |
| Rottlerin | 11.4 ± 1.70 | 4.01 ± 0.59 | 4.26 ± 0.50 | 1.27 ± 0.15 | 6.63 ± 0.93 |
| N-IND/MEN Control | 44.4 ± 3.39 | 15.9 ± 1.55 | 20.7 ± 1.39 | 1.56 ± 0.13 | 5.46 ± 0.41 |
| Nystatin | 26.1 ± 1.45 # | 9.3 ± 0.50 # | 13.3 ± 0.58 # | 1.67 ± 0.04 | 5.10 ± 0.11 |
| Dynasore | 30.6 ± 2.03 # | 10.9 ± 0.74 # | 14.2 ± 1.30 # | 1.54 ± 0.09 | 5.54 ± 0.27 |
| Rottlerin | 26.3 ± 0.98 # | 9.32 ± 0.25 # | 13.3 ± 0.68 # | 1.68 ± 0.05 | 5.08 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otake, H.; Yamaguchi, M.; Ogata, F.; Deguchi, S.; Yamamoto, N.; Sasaki, H.; Kawasaki, N.; Nagai, N. Energy-Dependent Endocytosis Is Responsible for Skin Penetration of Formulations Based on a Combination of Indomethacin Nanoparticles and l-Menthol in Rat and Göttingen Minipig. Int. J. Mol. Sci. 2021, 22, 5137. https://doi.org/10.3390/ijms22105137
Otake H, Yamaguchi M, Ogata F, Deguchi S, Yamamoto N, Sasaki H, Kawasaki N, Nagai N. Energy-Dependent Endocytosis Is Responsible for Skin Penetration of Formulations Based on a Combination of Indomethacin Nanoparticles and l-Menthol in Rat and Göttingen Minipig. International Journal of Molecular Sciences. 2021; 22(10):5137. https://doi.org/10.3390/ijms22105137
Chicago/Turabian StyleOtake, Hiroko, Mizuki Yamaguchi, Fumihiko Ogata, Saori Deguchi, Naoki Yamamoto, Hiroshi Sasaki, Naohito Kawasaki, and Noriaki Nagai. 2021. "Energy-Dependent Endocytosis Is Responsible for Skin Penetration of Formulations Based on a Combination of Indomethacin Nanoparticles and l-Menthol in Rat and Göttingen Minipig" International Journal of Molecular Sciences 22, no. 10: 5137. https://doi.org/10.3390/ijms22105137