Extrinsic or Intrinsic Apoptosis by Curcumin and Light: Still a Mystery
Abstract
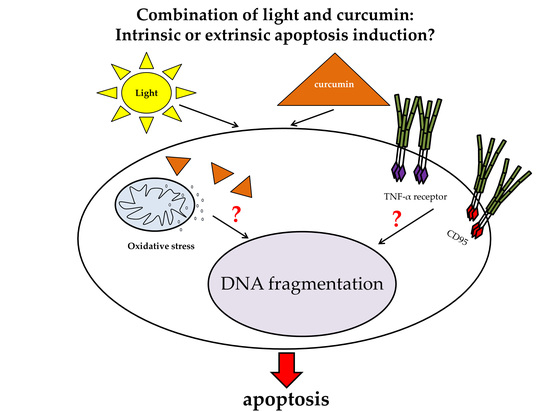
:1. Introduction
2. Results
2.1. Death Receptor Specific Apoptosis Induction Was Cell Species Dependent
2.2. Curcumin and Light Induced DNA Fragmentation Independent of the First Apoptosis Signal (FAS) Ligand and the TNF-α Receptors
2.3. Curcumin Increased the UVA Triggered H2O2 Generation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Identification of Death Receptor Agonist Susceptibility
4.2. Irradiation Regimen
4.3. DNA Fragmentation
4.4. Monitoring of the Cellular Redox Balence
4.5. Presentation of Data and Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TNF-α | tumor necrosis factor α |
| UVA | ultraviolet A |
| EGF | endothelial growth factor |
References
- Kadioglu, O.; Cao, J.; Saeed, M.E.; Greten, H.J.; Efferth, T. Targeting epidermal growth factor receptors and downstream signaling pathways in cancer by phytochemicals. Target. Oncol. 2015, 10, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Xu, Y.X.; Pindolia, K.R.; Janakiraman, N.; Noth, C.J.; Chapman, R.A.; Gautam, S.C. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp. Hematol. 1997, 25, 413–422. [Google Scholar] [PubMed]
- Zhu, L.; Han, M.B.; Gao, Y.; Wang, H.; Dai, L.; Wen, Y.; Na, L.X. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol. Med. Rep. 2015, 12, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, B.; Zhang, X.; Hazarika, P.; Aggarwal, B.B.; Duvic, M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: Potential role for STAT-3 and NF-kappaB signaling. J. Investig. Dermatol. 2010, 130, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Dujic, J.; Kippenberger, S.; Ramirez-Bosca, A.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A.; Hofmann, M. Curcumin in combination with visible light inhibits tumor growth in a xenograft tumor model. Int. J. Cancer 2009, 124, 1422–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calaf, G.M.; Ponce-Cusi, R.; Carrion, F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2018, 40, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutrit. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Hegge, A.B.; Bruzell, E.; Kristensen, S.; Tonnesen, H.H. Photoinactivation of Staphylococcus epidermidis biofilms and suspensions by the hydrophobic photosensitizer curcumin--effect of selected nanocarrier: Studies on curcumin and curcuminoides XLVII. Eur. J. Pharm. Sci. 2012, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, R.; Xie, Q.; Li, A.; Xiao, Y.; Li, K.; Liu, H.; Cui, D.; Chen, Y.; Wang, S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int. J. Nanomed. 2012, 7, 3667–3677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Teh, C.; Sreejith, S.; Zhu, L.; Kwok, A.; Fang, W.; Ma, X.; Nguyen, K.T.; Korzh, V.; Zhao, Y. Functional mesoporous silica nanoparticles for photothermal-controlled drug delivery in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8373–8377. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Godbole, M.M.; Misra, K. A plausible explanation for enhanced bioavailability of P-gp substrates in presence of piperine: Simulation for next generation of P-gp inhibitors. J. Mol. Model. 2013, 19, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.; Dobra, J.; Goerg, K.; Hoffmann, S.; Kippenberger, S.; Kaufmann, R.; Hofmann, M.; Bernd, A. Visible light is a better co-inducer of apoptosis for curcumin-treated human melanoma cells than UVA. PLoS ONE 2013, 8, e79748. [Google Scholar] [CrossRef] [PubMed]
- Dujic, J.; Kippenberger, S.; Hoffmann, S.; Ramirez-Bosca, A.; Miquel, J.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J. Investig. Dermatol. 2007, 127, 1992–2000. [Google Scholar] [CrossRef]
- Beyer, K.; Nikfarjam, F.; Butting, M.; Meissner, M.; König, A.; Ramirez Bosca, A.; Kaufmann, R.; Heidemann, D.; Bernd, A.; Kippenberger, S.; et al. Photodynamic Treatment of Oral Squamous Cell Carcinoma Cells with Low Curcumin Concentrations. J. Cancer 2017, 8, 1271–1283. [Google Scholar] [CrossRef] [Green Version]
- Megalathan, A.; Kumarage, S.; Dilhari, A.; Weerasekera, M.M.; Samarasinghe, S.; Kottegoda, N. Natural curcuminoids encapsulated in layered double hydroxides: A novel antimicrobial nanohybrid. Chem. Cent. J. 2016, 10, 35. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.H. Photosensitizer effect of curcumin on UVB-irradiated HaCaT cells through activation of caspase pathways. Oncol. Rep. 2007, 17, 537–540. [Google Scholar] [CrossRef]
- Kim, M.S.; Kang, H.J.; Moon, A. Inhibition of invasion and induction of apoptosis by curcumin in H-ras-transformed MCF10A human breast epithelial cells. Arch. Pharm. Res. 2001, 24, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.A.; Cheung, K.J., Jr.; Li, G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 2001, 271, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Hongmei, Z. Extrinsic and Intrinsic Apoptosis Signal Pathway Review. In Apoptosis and Medicine; Ntuli, T., Ed.; IntechOpen: London, UK, 2012; pp. 3–22. [Google Scholar]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Squires, M.S.; Hudson, E.A.; Howells, L.; Sale, S.; Houghton, C.E.; Jones, J.L.; Fox, L.H.; Dickens, M.; Prigent, S.A.; Manson, M.M. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem. Pharmacol. 2003, 65, 361–376. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, S.; Lin, J.; Zhang, Q.J.; Chen, A. The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARgamma in rat activated hepatic stellate cell in vitro. Lab. Investig. 2007, 87, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol. Urol. 2000, 4, 1–6. [Google Scholar] [PubMed]
- Choudhuri, T.; Pal, S.; Agwarwal, M.L.; Das, T.; Sa, G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002, 512, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Choudhuri, T.; Pal, S.; Das, T.; Sa, G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J. Biol. Chem. 2005, 280, 20059–20068. [Google Scholar] [CrossRef] [PubMed]
- Bielak-Mijewska, A.; Piwocka, K.; Magalska, A.; Sikora, E. P-glycoprotein expression does not change the apoptotic pathway induced by curcumin in HL-60 cells. Cancer Chemother. Pharmacol. 2004, 53, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Kim, Y.H.; Choi, Y.J.; Kim, D.G.; Lee, K.S.; Bae, J.H.; Min, D.S.; Chang, J.S.; Jeong, Y.J.; Lee, Y.H.; et al. Molecular mechanisms of curcumin-induced cytotoxicity: Induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis 2003, 24, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Wahl, H.; Tan, L.; Griffith, K.; Choi, M.; Liu, J.R. Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol. Oncol. 2007, 105, 104–112. [Google Scholar] [CrossRef]
- Deeb, D.; Jiang, H.; Gao, X.; Al-Holou, S.; Danyluk, A.L.; Dulchavsky, S.A.; Gautam, S.C. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J. Pharmacol. Exp. Ther. 2007, 321, 616–625. [Google Scholar]
- Schon, M.P.; Wienrich, B.G.; Drewniok, C.; Bong, A.B.; Eberle, J.; Geilen, C.C.; Gollnick, H.; Schon, M. Death receptor-independent apoptosis in malignant melanoma induced by the small-molecule immune response modifier imiquimod. J. Investig. Dermatol. 2004, 122, 1266–1276. [Google Scholar] [CrossRef]
- Min, K.J.; Woo, S.M.; Shahriyar, S.A.; Kwon, T.K. Elucidation for modulation of death receptor (DR) 5 to strengthen apoptotic signals in cancer cells. Arch. Pharm. Res. 2019, 42, 88–100. [Google Scholar] [CrossRef]
- Twomey, J.D.; Zhang, B. Circulating Tumor Cells Develop Resistance to TRAIL-Induced Apoptosis Through Autophagic Removal of Death Receptor 5: Evidence from an In Vitro Model. Cancers 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Peng, S.F.; Lee, C.Y.; Lu, C.C.; Tsai, S.C.; Shieh, T.M.; Wu, T.S.; Tu, M.G.; Chen, M.Y.; Yang, J.S. Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int. J. Oncol. 2013, 43, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Li, L.; Liu, M.Y.; Jin, X.B.; Mao, J.W.; Pu, Q.H.; Meng, M.J.; Chen, X.G.; Zhu, J.Y. Curcumin induces FasL-related apoptosis through p38 activation in human hepatocellular carcinoma Huh7 cells. Life Sci. 2013, 92, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Li, T.M.; Tsao, J.Y.; Fong, Y.C.; Tang, C.H. Curcumin induces cell apoptosis in human chondrosarcoma through extrinsic death receptor pathway. Int. Immunopharmacol. 2012, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Xu, C.; Keum, Y.S.; Reddy, B.; Conney, A.; Kong, A.N. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with beta-phenylethyl isothiocyanate and curcumin. Carcinogenesis 2006, 27, 475–482. [Google Scholar] [CrossRef]
- Chresta, C.M.; Arriola, E.L.; Hickman, J.A. Apoptosis and cancer chemotherapy. Behring Inst. Mitt. 1996, 232–240. [Google Scholar]
- Thulasiraman, P.; Garriga, G.; Danthuluri, V.; McAndrews, D.J.; Mohiuddin, I.Q. Activation of the CRABPII/RAR pathway by curcumin induces retinoic acid mediated apoptosis in retinoic acid resistant breast cancer cells. Oncol. Rep. 2017, 37, 2007–2015. [Google Scholar] [CrossRef] [Green Version]
- Labbozzetta, M.; Notarbartolo, M.; Poma, P.; Maurici, A.; Inguglia, L.; Marchetti, P.; Rizzi, M.; Baruchello, R.; Simoni, D.; D’Alessandro, N. Curcumin as a possible lead compound against hormone-independent, multidrug-resistant breast cancer. Ann. N. Y. Acad. Sci. 2009, 1155, 278–283. [Google Scholar] [CrossRef]
- Chatterjee, S.J.; Pandey, S. Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy. Cancer Biol. Ther. 2011, 11, 216–228. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Ganapathy, S.; Chen, Q.; Srivastava, R.K. Curcumin sensitizes TRAIL-resistant xenografts: Molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol. Cancer 2008, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.R.; Eid, R.; Boucher, E.; Miller, K.A.; Mandato, C.A.; Greenwood, M.T. Stress is an agonist for the induction of programmed cell death: A review. Biochim. Biophys. Acta 2019. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhu, R.; He, X.; Wang, J.; Wang, M.; Qian, Y.; Wang, S. Enhanced photocytotoxicity of curcumin delivered by solid lipid nanoparticles. Int. J. Nanomed. 2017, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Rahmani, F.; Aghaee-Bakhtiari, S.H.; Avan, A.; Khazaei, M. Phytosomal curcumin elicits anti-tumor properties through suppression of angiogenesis, cell proliferation and induction of oxidative stress in colorectal cancer. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Patwardhan, R.S.; Pal, D.; Singh, B.; Sharma, D.; Kutala, V.K.; Sandur, S.K. Mitochondrial targeted curcumin exhibits anticancer effects through disruption of mitochondrial redox and modulation of TrxR2 activity. Free Rad. Biol. Med. 2017, 113, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Salehi, E.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Najafi, M.; Farhood, B.; Rosengren, R.J.; Sahebkar, A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.K.; Paul, M.; Paul, S. Curcumin induces caspase mediated apoptosis in JURKAT cells by disrupting the redox balance. Asian Pac. J. Cancer Prev. 2014, 15, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zöller, N.; Valesky, E.; Butting, M.; Hofmann, M.; Kippenberger, S.; Bereiter-Hahn, J.; Bernd, A.; Kaufmann, R. Clinical application of a tissue-cultured skin autograft: An alternative for the treatment of non-healing or slowly healing wounds? Dermatology 2014, 229, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Golinski, P.; Menke, H.; Hofmann, M.; Valesky, E.; Butting, M.; Kippenberger, S.; Bereiter-Hahn, J.; Bernd, A.; Kaufmann, R.; Zöller, N.N. Development and Characterization of an Engraftable Tissue-Cultured Skin Autograft: Alternative Treatment for Severe Electrical Injuries. Cells Tissues Organs 2014, 200, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zöller, N.N.; Kippenberger, S.; Thaci, D.; Mewes, K.; Spiegel, M.; Sattler, A.; Schultz, M.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A. Evaluation of beneficial and adverse effects of glucocorticoids on a newly developed full-thickness skin model. Toxicol. in Vitro 2008, 22, 747–759. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laubach, V.; Kaufmann, R.; Bernd, A.; Kippenberger, S.; Zöller, N. Extrinsic or Intrinsic Apoptosis by Curcumin and Light: Still a Mystery. Int. J. Mol. Sci. 2019, 20, 905. https://doi.org/10.3390/ijms20040905
Laubach V, Kaufmann R, Bernd A, Kippenberger S, Zöller N. Extrinsic or Intrinsic Apoptosis by Curcumin and Light: Still a Mystery. International Journal of Molecular Sciences. 2019; 20(4):905. https://doi.org/10.3390/ijms20040905
Chicago/Turabian StyleLaubach, Vesselina, Roland Kaufmann, August Bernd, Stefan Kippenberger, and Nadja Zöller. 2019. "Extrinsic or Intrinsic Apoptosis by Curcumin and Light: Still a Mystery" International Journal of Molecular Sciences 20, no. 4: 905. https://doi.org/10.3390/ijms20040905