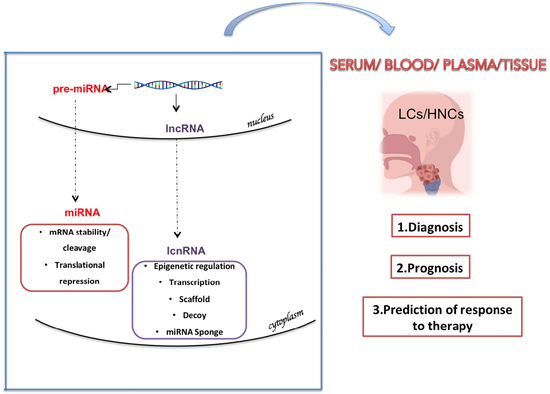
Long Non-coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours
Abstract
:1. Introduction
2. Biological Knowledge of Laryngeal and Head and Neck Cancers
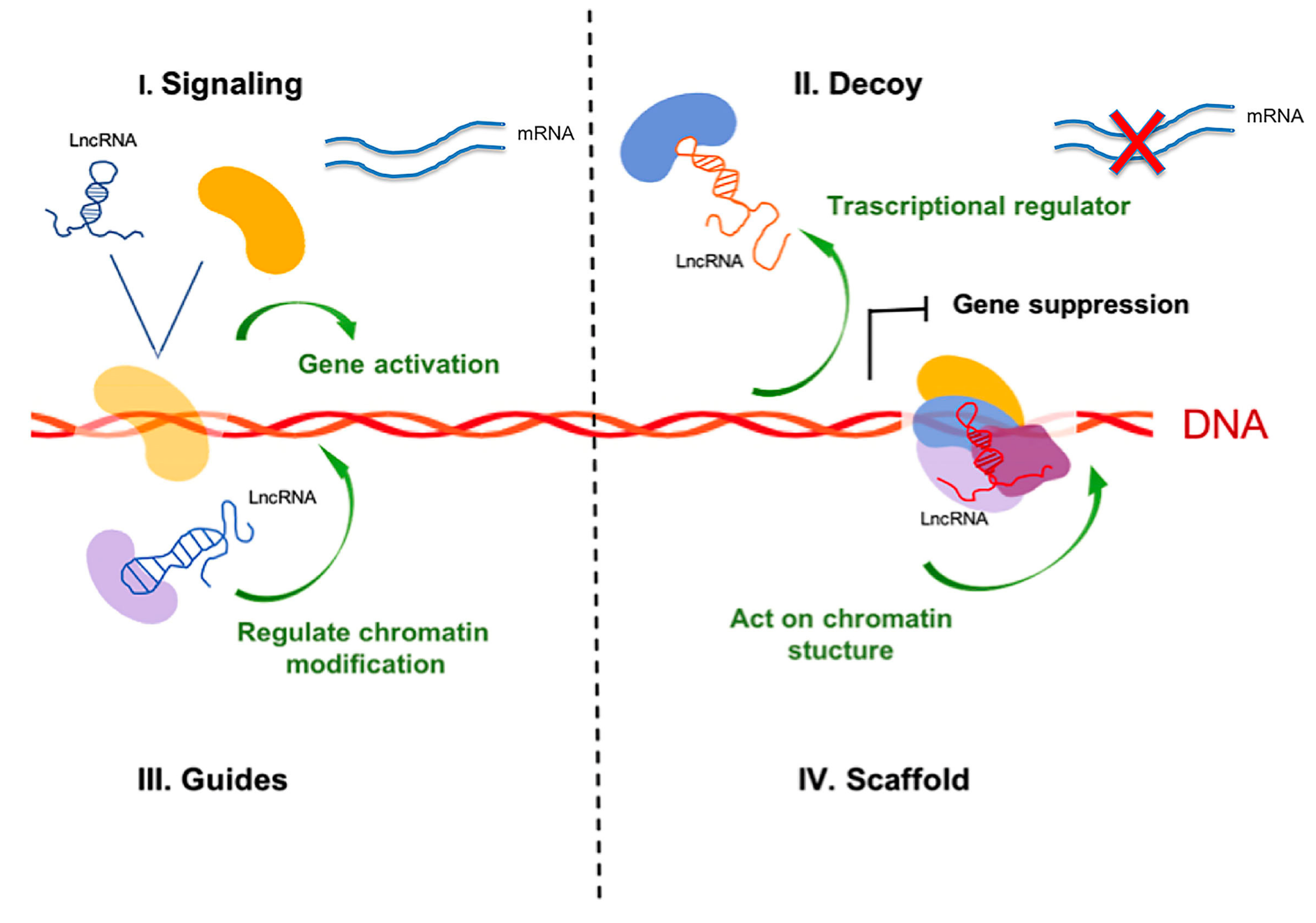
3. Classification and Characteristics of Non-coding RNAs
4. Prognosis Associated lncRNAs in HNC
4.1. HOTAIR
4.2. UCA1
4.3. FOXC1
4.4. AFAP1-AS1
4.5. HNF1A-AS
4.6. ROR
4.7. LET
4.8. PlncRNA-1
4.9. GAS8-AS1
4.10. ADAMTS9-AS2
4.11. ESCCAL-1
4.12. PVT1
4.13. PTCSC2- PTCSC3
4.14. FIRRE
4.15. H19
4.16. MEG3
4.17. MALAT-1
4.18. LOC541471
5. LncRNAs in Laryngeal Cancer
5.1. DGCR5
5.2. PCAT19
5.3. H19
5.4. ST7-AS1
5.5. NEAT1
5.6. UCA1
5.7. NF90
5.8. LINC00668
5.9. SNHG1
5.10. TUG1
5.11. MALAT-1
5.12. LINC00460 and LINC00520
5.13. LOC554202
5.14. Dleu2
5.15. HOXA11-AS
5.16. CCAT1
5.17. RP11-169D4.1
5.18. LINC00261
5.19. NR027340-SOX2-OT
5.20. HOTAIR
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| HNC | head and neck cancer |
| HNSCC | head and neck squamous cell carcinoma |
| HPV | human papillomavirus |
| LncRNAs | long non-coding RNAs |
| OSCC | oral squamous cell carcinoma |
| LSCC | laryngeal squamous cell carcinoma |
| TSCC | tongue squamous cell carcinoma |
| HSCC | hypopharyngeal squamous cell carcinoma |
| NPC | nasopharyngeal carcinoma |
| TC | thyroid cancer |
| PTC | papillary thyroid cancer |
References
- Marur, S.; Forastiere, A.A. Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belcher, R.; Hayes, K.; Fedewa, S.; Chen, A.Y. Current treatment of head and neck squamous cell cancer. J. Surg. Oncol. 2014, 110, 551–574. [Google Scholar] [CrossRef] [PubMed]
- Mastronikolis, N.S.; Papadas, T.A.; Goumas, p.d.; Triantaphyllidou, I.E.; Theocharis, D.A.; Papageorgakopoulou, N.; Vynios, D.H. Head and neck: Laryngealtumors: An overview. Atlas. Genet. Cytogenet. Oncol. Haematol. 2009, 13, 888–893. [Google Scholar] [CrossRef]
- Ricciardiello, F.; Caraglia, M.; Iorio, B.; Abate, T.; Boccellino, M.; Colella, G.; Oliva, F.; Ferrise, P.; Zappavigna, S.; Faenza, M.; et al. Aggressiveness pattern and second primary tumor risk associated with basaloid squamous cell carcinoma of the larynx. Oncotarget 2017, 8, 95791–95798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koontongkaew, S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Deng, Y.F. Long non-coding RNAs as novel biomarkers and therapeutic targets in head and neck cancers. Int. J.Clin. Exp.Pathol. 2014, 7, 1286–1292. [Google Scholar]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. Lincrnas: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef]
- Ziegler, C.; Kretz, M. The more the merrier-complexity in long non-coding RNA loci. Front. Endocrinol (Lausanne) 2017, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Pinto, F.; Auletta, A.; Giordano, A.; Giovane, A.; Settembre, G.; Boccellino, M.; Boffo, S.; Di Carlo, A.; di Domenico, M. The enigmatic role of matrix metalloproteinases in epithelial-to-mesenchymal transition of oral squamous cell carcinoma: Implications and nutraceutical aspects. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–887. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, M.; Eskander, A.; Irish, J.; Gullane, P.; Gilbert, R.; Brown, D.; de Almeida, J.R.; Urbach, D.R.; Goldstein, D.P. Evolving trends in head and neck cancer epidemiology: Ontario, Canada 1993–2010. Head Neck 2017, 39, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Marks, J.E.; Phillips, J.L.; Menck, H.R. The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer 1998, 83, 582–588. [Google Scholar] [CrossRef]
- Wei, W.; Sham, J.S. Nasopharyngeal carcinoma. Lacet 2005, 365, 2041–2054. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med. 2018, 7, 241. [Google Scholar] [CrossRef]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Ohashi, S.; Miyamoto, S.; Kikuchi, O.; Goto, T.; Amanuma, Y.; Muto, M. Recent Advances from Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology 2015, 149, 1700–1715. [Google Scholar] [CrossRef]
- Mercer, T.; Mattick, J. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Vanacore, D.; Zappavigna, S.; Cavaliere, C.; Rossetti, S.; D’Aniello, C.; Chieffi, P.; Amler, E.; Buonerba, C.; di Lorenzo, G.; et al. Testicular cancer from diagnosis to epigenetic factors. Oncotarget 2017, 18, 104654–104663. [Google Scholar] [CrossRef] [PubMed]
- Montanari, M.; la Mantia, E.; Piscitelli, R.; Nocerino, F.; Cappuccio, F.; Grimaldi, G.; Izzo, A.; Castaldo, L.; Pepe, M.F.; Malzone, M.G.; et al. Micrornas in prostate cancer: An overview. Oncotarget 2017, 25, 50240–50251. [Google Scholar]
- Lamberti, M.; Capasso, R.; Lombardi, A.; di Domenico, M.; Fiorelli, A.; Feola, A.; Perna, A.F.; Santini, M.; Caraglia, M.; Ingrosso, D. Two Different Serum MiRNA Signatures Correlate with the Clinical Outcome and Histological Subtype in Pleural Malignant Mesothelioma Patients. PLoS ONE 2015, 11, e0135331. [Google Scholar] [CrossRef] [PubMed]
- Balas, M.M.; Johnson, A.M. Exploring the mechanisms behind long noncoding RNAs and cancer. Noncoding RNA Res. 2018, 3, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, K.K.; Zhang, J.; Xiao, B.; Huang, Z.; Ju, C.; Sun, J.; Zhang, F.; Lv, X.B.; Huang, G. The decade of exosomal long RNA species: An emerging cancer antagonist. Mol. Cancer 2018, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Alaia, C.; Misso, G.; Cossu, A.M.; Facchini, G.; Piscitelli, R.; Quagliuolo, L.; Caraglia, M. Gene interference strategies as a new tool for the treatment of prostate cancer. Endocrine 2015, 49, 588–605. [Google Scholar] [CrossRef] [PubMed]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef] [Green Version]
- Guttman, M.; Rinn, J.L. Modular regulatory principles of large non-coding RNAs. Nature 2012, 482, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, M.; Yuan, C.; Han, T.; Cui, J.; Jiao, F.; Wang, L. A new feedback loop between the high MALAT-1 and the low miR-200c-3p promotes cell migration and invasion of in-pancreatic ductal adenocarcinoma and is predictive of poor prognosis. BMC Cancer 2018, 18, 1032. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.H.; Ni, P.; Gu, Z.; Qiao, Y.; Chen, N.; Sun, F.; Fan, Q. CREB up-regulates long non-coding RNA, HULC expression through interaction withmicroRNA-372 in liver cancer. Nucleic Acids Res. 2010, 38, 5366–5383. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-codingRNAs as targets for anticancerdrugdevelopment. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Hajjari, M.; Salavaty, A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Biol. Med. 2015, 12, 1–9. [Google Scholar] [PubMed]
- Luo, Z.F.; Zhao, D.; Li, X.Q.; Cui, Y.X.; Ma, N.; Lu, C.X.; Liu, M.Y.; Zhou, Y. Clinical significance of HOTAIR expression in colon cancer. World J.Gastroenterol. 2016, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, H. Expression of long noncoding RNA-HOX transcript antisense intergenic RNA in oral squamous cell carcinoma and effect on cell growth. Tumour Biol. 2015, 36, 8573–8578. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.M.; Lu, Y.F.; Hu, B.G.; Liang, W.C.; Zhu, X.; Yang, H.D.; Li, G.; Zhang, J.F. Long noncoding RNA hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling pathways. Oncotarget 2016, 74712–74723. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wan, Q.; Wang, W.; Mai, L.; Sha, L.; Mashrah, M.; Lin, Z.; Pan, C. LncRNA ADAMTS9-AS2 promotes tongue squamous cell carcinoma proliferation, migration and EMT via the miR-600/EZH2 axis. Biomed. Pharmacother. 2019, 112, 108719. [Google Scholar] [CrossRef] [PubMed]
- Bo, H.; Gong, Z.; Zhang, W.; Li, X.; Zeng, Y.; Liao, Q.; Chen, P.; Shi, L.; Lian, Y.; Jing, Y.; et al. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget 2015, 6, 20404–20418. [Google Scholar]
- Cui, Z.; Ren, S.; Lu, J.; Wang, F.; Xu, W.; Sun, Y.; Wei, M.; Chen, J.; Gao, X.; Xu, C.; et al. The prostate cancer-up-regulated long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation through reciprocal regulation of androgen receptor. Urol.Oncol. 2013, 31, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Wu, Q.Q.; Li, S.Q.; Chen, F.J.; Tuo, L.; Xie, H.W.; Tong, Y.S.; Ji, L.; Zhou, G.Z.; Cao, G.; et al. Upregulation of the long non-coding RNA PlncRNA-1 promotes esophageal squamous carcinoma cell proliferation and correlates with advanced clinical stage. Dig. Dis. Sci. 2014, 59, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Alaia, C.; Boccellino, M.; Zappavigna, S.; Amler, E.; Quagliuolo, L.; Rossetti, S.; Facchini, G.; Caraglia, M. Ipilimumab for the treatment of metastatic prostate cancer. Expert Opin. Biol.Ther. 2018, 18, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wu, W.; Shi, F.; Dalmolin, R.J.; Yan, M.; Tian, F.; Chen, X.; Chen, G.; Cao, W. Prediction of long noncoding RNA functions with co-expression network in esophageal squamous cell carcinoma. BMC Cancer 2015, 24, 15–168. [Google Scholar] [CrossRef] [PubMed]
- Hacisuleyman, E.; Goff, L.A.; Trapnell, C.; Williams, A.; Henao-Mejia, J.; Sun, L.; McClanahan, P.; Hendrickson, D.G.; Sauvageau, M.; Kelley, D.R.; et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat. Struct. Mol. Biol. 2014, 21, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Nijiro, N.; Abba, M.C.; Gutkinda, J.S. Unraveling the Oral Cancer lncRNAome: Identification of Novel lncRNAs Associated with Malignant Progression and HPV Infection. Oral Oncol. 2016, 59, 58–66. [Google Scholar]
- Kong, X.P.; Yao, J.; Luo, W.; Feng, F.K.; Ma, J.T.; Ren, Y.P.; Wang, D.L.; Bu, R.F. The expression and functional role of a FOXC1 related mRNA-lncRNA pair in oral squamous cell carcinoma. Mol. Cell. Biochem. 2014, 394, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, W.; Zhou, L.; Ge, M.; Zhang, B.; Yang, X.; Xiong, X.; Fu, G.; Zhang, J.; Nie, X.; Li, H.; et al. Whole exome sequencing identifies lncRNA GAS8-AS1 and LPAR4 as novel papillary thyroid carcinoma driver alternations. Hum. Mol. Genet. 2016, 25, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Y.; Yang, X.; Wu, X.; He, X. Long noncoding RNA H19 regulates EZH2 expression by interacting with miR-630 and promotes cell invasion in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2016, 13, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; Wu, Q.; Jin, C.S.; Yuan, H.J.; Cheng, J.Z. Long non-coding RNA HNF1A-AS is upregulated and promotes cell proliferation and metastasis in nasopharyngeal carcinoma. Cancer Biomark. 2016, 16, 291–300. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, H.; Li, L.; Zhang, S.; Liu, K.; Liu, Y.; Yang, C. Long noncoding RNA-LET, which is repressed by EZH2, inhibits cell proliferation and induces apoptosis of nasopharyngeal carcinoma cell. Med. Oncol. 2015, 32, 226. [Google Scholar] [CrossRef]
- Li, L.; Gu, M.; You, B.; Shi, S.; Shan, Y.; Bao, L.; You, Y. Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci. 2016, 107, 1215–1222. [Google Scholar] [CrossRef]
- Wu, H.; Yu, D.H.; Wu, M.H.; Huang, T. Long non-coding RNA LOC541471: A novel prognostic biomarker for head and neck squamous cell carcinoma. Oncol. Lett. 2019, 17, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhang, S.; Wang, Y.; Shen, S.; Wang, F.; Hao, Y.; Li, Y.; Zhang, B.; Zhou, Y.; Yang, H. Long non-coding RNA MALAT-1 modulates metastatic potential of tongue squamous cell carcinomas partially through the regulation of small proline rich proteins. BMC Cancer 2016, 16, 706. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.F.; Wei, S.B.; Gan, Y.H.; Guo, Y.; Gong, K.; Mitchelson, K.; Cheng, J.; Yu, G.Y. Expression, regulation and roles of miR-26a and MEG3 in tongue squamous cell carcinoma. Int. J. Cancer 2014, 135, 2282–2293. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, W.; Liyanarachchi, S.; Jendrzejewski, J.; Srinivas, M.; Davuluri, R.V.; Nagy, R.; de la Chapelle, A. Genetic predisposition to papillary thyroid carcinoma: Involvement of FOXE1, TSHR, and a novel lincRNA gene, PTCSC2. J. Clin. Endocrinol. Metab. 2015, 100, E164–E172. [Google Scholar] [CrossRef] [PubMed]
- Jendrzejewski, J.; He, H.; Radomska, H.S.; Li, W.; Tomsic, J.; Liyanarachchi, S.; Davuluri, R.V.; Nagy, R.; de la Chapelle, A. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc. Natl. Acad. Sci. USA 2012, 109, 8646–8651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Chen, J.; Feng, J.; Wang, J. Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR). Tumour Biol. 2016, 37, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wu, L.; Wang, L.; Yang, Y.; Meng, Y.; Yang, H. Increased expression of the long non-coding RNA UCA1 in tongue squamous cell carcinomas: A possible correlation with cancer metastasis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 89–95. [Google Scholar] [CrossRef]
- Tang, T.; Shan, G. DGCR5 promotes cancer stem cell-like properties of radioresistant laryngeal carcinoma cells by sponging miR-506 via Wnt pathway. J. Cell. Physiol. 2019, 234, 18423–18431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, H. Long non-coding RNA CCAT1/miR-218/ZFX axis modulates the progression of laryngeal squamous cell cancer. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Qu, L.; He, G.; Tian, L.; Li, L.; Zhou, H.; Jin, Q.; Ren, J.; Wang, Y.; Wang, J.; et al. Regulation of laryngeal squamous cell cancer progression by the lncRNA H19/miR-148a-3p/DNMT1 axis. Oncotarget 2016, 7, 11553–11566. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Feng, J.; Wu, T.; Wang, Y.; Sun, Y.; Ren, J.; Liu, M. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am. J. Pathol. 2013, 182, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Jin, M.; Yang, L.; Sun, C.; Wang, P.; Li, Y.; Tian, L.; Liu, M.; Sun, Y. Expression of long non-coding RNA HOXA11-AS is correlated with progression of laryngeal squamous cell carcinoma. Am. J. Transl. Res. 2018, 10, 573–580. [Google Scholar] [PubMed]
- Ge, S.S.; Wu, Y.Y.; Gao, W.; Zhang, C.M.; Hou, J.; Wen, S.X.; Wang, B.Q. Expression of long non-coding RNA LINC00460 in laryngeal squamous cell carcinoma tissue and its clinical significance. Lin Chung Er Bi Yan HouTou Jing WaiKeZaZhi 2018, 32, 18–22. [Google Scholar]
- Wu, Y.Y.; Gao, W.; Zhang, Y.L.; Niu, M.; Cui, J.J.; Xiang, C.X.; Sang, J.W.; Wen, S.X.; Wang, B.Q. Expression and clinical significance of long non-coding RNA LINC00520 in laryngeal squamous cell carcinoma. Lin Chung Er Bi Yan HouTou Jing WaiKeZaZhi 2018, 32, 91–95. [Google Scholar]
- Zhao, L.; Cao, H.; Chi, W.; Meng, W.; Cui, W.; Guo, W.; Wang, B. Expression profile analysis identifies the long non-coding RNA landscape and the potential carcinogenic functions of LINC00668 in laryngeal squamous cell carcinoma. Gene 2019, 687, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.Z.; Xiao, Z.C.; Song, Y.X.; Li, W.; Tan, G.L. Long non-coding RNA Dleu2 affects proliferation, migration and invasion ability of laryngeal carcinoma cells through triggering miR-16-1 pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1963–1970. [Google Scholar] [PubMed]
- Zhang, C.M.; Gao, W.; Wu, Y.Y.; Zhao, Q.L.; Chen, B.; Liu, Q.Q.; Li, W.Y.; Wen, S.X.; Wang, B.Q. The expression of long non-coding RNA LINC00261 in laryngeal carcinoma tissue and their clinical significance. Lin Chung Er Bi Yan HouTou Jing WaiKeZaZhi 2017, 31, 68–71. [Google Scholar]
- Yang, S.; Wang, J.; Ge, W.; Jiang, Y. Long non-coding RNA LOC554202 promotes laryngeal squamous cell carcinoma progression through regulating miR-31. J. Cell.Biochem. 2018, 119, 6953–6960. [Google Scholar] [CrossRef]
- Han, Y.; Chen, D.; Li, H.; Wang, X.; Zhang, M.; Yang, Y. Long chain non-coding RNA MALAT-1 gene knockdown inhibits growth and migration and promotes apoptosis of human laryngeal squamous cell carcinoma Hep-2 cells in vitro. Nan Fang Yi Ke Da XueXueBao 2018, 38, 923–930. [Google Scholar]
- Wang, P.; Wu, T.; Zhou, H.; Jin, Q.; He, G.; Yu, H.; Xuan, L.; Wang, X.; Tian, L.; Sun, Y.; et al. Long noncoding RNA NEAT1 promotes laryngeal squamous cell cancer through regulating miR-107/CDK6 pathway. J. Exp.Clin. Cancer Res. 2016, 35, 22. [Google Scholar] [CrossRef]
- Feng, L.; Wang, R.; Lian, M.; Ma, H.; He, N.; Liu, H.; Wang, H.; Fang, J. Integrated Analysis of Long Noncoding RNA and mRNA Expression Profile in Advanced Laryngeal Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0169232. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Guo, J.; Zhang, W. LncRNA PCAT19 promotes the proliferation of laryngocarcinoma cells via modulation of the miR-182/PDK4 axis. J. Cell.Biochem. 2019, 120, 12810–12821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lv, K.; Li, Z.H.; Wu, J.; Gao, W.; Wong, T.S.; Luo, J.; Qin, H.; Wang, B.; Fu, Q.; et al. Functional significance of the long non-coding RNA RP11-169D4.1 as a metastasis suppressor in laryngeal squamous cell carcinoma by regulating CDH1. Oncol. Rep. 2017, 38, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Xu, J.; Gong, L.; Jiang, B.; Zhao, W. The long noncoding RNA ST7-AS1 promotes laryngeal squamous cell carcinoma by stabilizing CARM1. Biochem.Biophys. Res.Commun. 2019, 512, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Dinger, M.E.; Mercer, T.R.; Mehler, M.F. RNA regulation of epige-netic processes. Bioessays 2009, 31, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Yang, Y.; Zha, D.; Yue, B.; Qiu, J.; Zhang, C. Overexpression of lncRNAsnaR is correlated with progression and predicts poor survival of laryngeal squamous cell carcinoma. J. Cell Biochem. 2018. [Google Scholar] [CrossRef]
- Gao, L.; Cao, H.; Cheng, X. A positive feedback regulation between long noncoding RNA SNHG1 and YAP1 modulates growth and metastasis in laryngeal squamous cell carcinoma. Am. J. Cancer Res. 2018, 8, 1712–1724. [Google Scholar] [PubMed]
- Zhang, Z.; Wang, X.; Cao, S.; Han, X.; Wang, Z.; Zhao, X.; Liu, X.; Li, G.; Pan, X.; Lei, D. The Long Noncoding RNA TUG1 Promotes Laryngeal Cancer Proliferation and Migration. Cell. Physiol. Biochem. 2018, 49, 2511–2520. [Google Scholar] [CrossRef]
- Sun, S.; Gong, C.; Yuan, K. LncRNA UCA1 promotes cell proliferation, invasion and migration of laryngeal squamous cell carcinoma cells by activating Wnt/β-catenin signaling pathway. Exp. Ther. Med. 2019, 17, 1182–1189. [Google Scholar] [CrossRef]
- Tsai, M.C.; Spitale, R.C.; Chang, H.Y. Long intergenicnoncoding RNAs: New links in cancer progression. Cancer Res. 2011, 71, 3–7. [Google Scholar] [CrossRef]
- Matouk, I.J.; Mezan, S.; Mizrahi, A.; Ohana, P.; Abu-Lail, R.; Fellig, Y.; Degroot, N.; Galun, E.; Hochberg, A. The oncofetalH19 RNA connection: Hypoxia, p53 and cancer. Biochim. Biophysica. Acta 2010, 1803, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-codingRNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Kogo, R.; Shimamura, T.; Mimori, K.; Kawahara, K.; Imoto, S.; Sudo, T.; Tanaka, F.; Shibata, K.; Suzuki, A.; Komune, S.; et al. Long non-coding RNA HOTAIR regulates polycomb-dependent chromatin modificationand is associated with poor prognosis in colorectal cancers. Cancer Res. 2011, 71, 6320–6326. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jutooru, I.; Chadalapaka, G.; Johnson, G.; Frank, J.; Burghardt, R.; Kim, S.; Safe, S. HOTAIR isa negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 2011, 32, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, L. New Insights into Long Non-Coding RNA MALAT1 in Cancer and Metastasis. Cancers (Basel) 2019, 11, 216. [Google Scholar] [CrossRef]
- Mitra, R.; Chen, X.; Greenawalt, E.J.; Maulik, U.; Jiang, W.; Zhao, Z.; Eischen, C.M.; Chen, X.; Greenawalt, E.J.; Maulik, U.; et al. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat. Commun. 2017, 17, 1604. [Google Scholar] [CrossRef]

| lncRNA | Description | Cancer Type | Expression | Function and Mechanism | Application | Ref | |
|---|---|---|---|---|---|---|---|
| ADAMTS9-AS2 | - | TSCC | UP | Regulates miR-6010/EZH2 promoting cell migration and invasion via ETM processes | Diagnostic and prognostic biomarker | [37] | |
| AFAP1-AS1 | LncRNA actin filament-associated protein 1 antisense RNA1 | NPC | UP | Affects the expression of cytoskeletally-regulated proteins via inhibiting the Rho/Rac signaling pathway. Promotes cell migration and metastasis | Diagnostic and prognostic biomarker | [38] | |
| CBR3-AS1 | PlncRNA-1 prostate cancer-up-regulated long noncoding RNA1 | ESCC | UP | Promotes cell proliferation and correlates with advanced clinical stage and lymph node metastasis | Diagnostic and prognostic biomarker | [39,40,41] | |
| ESCCAL-1 | - | ESCC | UP | Knockdown ESCCAL-1 expression increases apoptosis and reduces invasion. | Diagnostic biomarker | [42] | |
| FIRRE | LncRNA functional intergenic repeating RNA element | HNSCC | UP | Interacts with the nuclear matrix factor hnRNPU across at least five distinct trans-chromosomal loci | Roles in cell physiology and nuclear architecture | [43,44] | |
| FOXCUT | FOXC1 upstream transcript | NPC OSCC | UP | Inhibits cell proliferation and cell migration | Diagnostics biomarker and therapeutics target | [45] | |
| GAS8-AS1 | LncRNA growth arrest-specific 8-antisense RNA 1 | PTC | DOWN | Inhibits cell viability | Diagnostic and therapeutic target | [46] | |
| H19 | - | NPC HNSCC | UP | Promotes cell invasion by inhibiting the activity of miR-630 and enhancing the expression of zeste homolog2 (EZH2). MiR-675 is correlated with H19. | Prognostic biomarker | [47]. | |
| HNF1A-AS | Hepatocyte nuclear factor 1A antisense RNA | NPC | UP | Increases mesenchymal proteins N-cadherin and vimentin levels. Reduces the epithelial E-cadherin protein levels. Promotes cell cycle progression, tumor cell proliferation, and migration. | Therapeutics target | [48] | |
| HOTAIR | Homeobox transcript antisense RNA | NPC | UP | Promotes cancer growth, angiogenesis and metastasis | Diagnostic and prognostic biomarker | [33,34,35,36] | |
| LncRNA-LET | LncRNA-Low Expression in tumor | NPC | DOWN | Associates with cell proliferation, lymph node metastasis. Low levels of LET expression are induced by EZH2-mediated H3K27 histone methylation in Let promoter region | Diagnostic biomarker | [49] | |
| LncRNA-ROR | - | NPC | UP | Promotes cell proliferation, metastasis and invasion ability by inducing an EMT phenotype. | Therapeutic target | [50] | |
| LOC541471 | HNSCC | UP | Increases lymph node metastasis and perineural invasion | Prognostic factor | [51] | ||
| MALAT-1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 | TSCC | UP | Reduces miR-124 expression and increases growth and metastasis of TSCC cells via targeting jagged 1 (JAG1). Induces EMT, promotes migration ad invasion and inhibits apoptosis of TSCC cells. | Diagnostic and prognostic biomarker | [52] | |
| MEG3 | Maternally expressed Gene 3 | TSCC | DOWN | Inhibits cell proliferation and the cell cycle, promotes cell apoptosis; DNMT3B is the intermediary by witch miR-26a regulates MEG3 expression. | Diagnostic and prognostic biomarker | [53] | |
| PTCSC2 | - | PTC | DOWN | Influences genes expression and cell cycle | - | [54] | |
| PTCSC3 | - | PTC | DOWN | Inhibits cell grow, influences the expression of genes involved in DNA replication, recombination and repair; cellular movement; tumor morphology and cell death. | Diagnostic biomarker | [55] | |
| PVT1 | - | TC | UP | Promotes cell proliferation and cell cycle progression in TC by recruiting EZH2 and regulating TSHR | Diagnostic biomarker | [56] | |
| UCA1 | Urothelial carcinoma- associated 1 | TSCC | UP | Promotes migration | Diagnostic and therapeutic strategy | [57] | |
| lncRNA | Description | Cancer Type | Expression | Function and Mechanism | Application | Ref |
|---|---|---|---|---|---|---|
| CCAT1 | Colon cancer-associated transcript-1 | LSCC | UP | Activates cancer cell proliferation, migration and invasion | Diagnostic and prognostic biomarker | [59] |
| DGCR5 | LncRNA Di George syndromecriticalregion gene5 | LSCC | UP | Induces cancer stem cells-like properties by sponging miR-506 trough Wnt signaling activation | Diagnostic biomarker | [58] |
| H19 | - | LSCC | UP | Increases DNA methylation by repressing miR-148a-3p | Prognostic biomarker | [60] |
| HOTAIR | Homeobox transcript antisense RNA | LSCC | UP | Promotes cancer growth, angiogenesis and metastasis | Diagnostic and prognostic biomarker | [61] |
| HOXA11-AS | HOXA11 antisense RNA | LSCC | UP | Promotes cancer growth, angiogenesis and neck nodal metastasis | Diagnostic biomarker and therapeutic target | [62] |
| LINC00460 | Long intergenic non-protein coding RNA 460 | LSCC | UP | Contributes to the carcinogenesis and development of LSCC | - | [63] |
| LINC00520 | Long intergenic non-protein coding RNA 520 | LSCC | UP | Induces metastasis. Promotes cell growth, cell cycle and cell invasion. Suppresses miR-31 expression and promotes RhoA expression. | Diagnostic and prognostic biomarker | [64] |
| LIN00668 | Long intergenic non-proteincoding RNA 668 | LSCC | UP | Promotes cell proliferation, migration and invasion. Associates with age, pathological differentiation degree, T stage and Lymph node metastasis. | Diagnostic and prognostic biomarker | [65] |
| LncRNA Dleu2 | - | LSCC | DOWN | Influences the proliferation, migration, and invasion of laryngeal cancer cells through miR-16-1 | Diagnostic biomarker and therapeutic target | [66] |
| LIN00261 | - | LSCC | DOWN | Contributes to the carcinogenesis and development of LSCC | Therapeutic target | [67] |
| LOC554202 | - | LSCC | UP | Promotes cell growth, cell cycle and invasion; suppresses miR-31 expression and promotes RhoA expression | - | [68] |
| MALAT-1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 | LSCC | UP | Promotes cell proliferation and inhibits apoptosis | Diagnostic and prognostic biomarker | [69] |
| NEAT1 | Nuclear enrich abundant transcript 1 | LSCC | UP | Promotes tumor growth and cell cycle progression in LSCC by regulating miR-107/CD46 pathway | - | [70] |
| NR027340 | - | LSCC | UP | - | Diagnostic biomarker and target therapy | [71] |
| PCAT 19 | - | LSCC | UP | Decreases cells proliferation, inhibits glycolysis by modulating the mir-182/pdk4 axis | Diagnostic biomarker | [72] |
| RP11-169 D4.1-001 | - | LSCC | DOWN | Increases lymph node metastasis in patients. Suppresses proliferation and promote apoptosis. Inhibits migration, invasion and epithelial-mesenchymal transitions (EMT) | Diagnostic and prognostic biomarker | [73] |
| ST7-AS1 | LncRNA suppressor of tumorigenicity 7 antisense RNA 1 | LCSS | UP | Promotes migration. Interacts with CARMI (coactivator-associated arginine methyltransferase) protecting it from ubiquitin-dependent degradation | Diagnostic biomarker | [74] |
| SOX2-OT | - | LSCC | UP | - | Diagnostic and prognosticbiomarker | [75] |
| SNAR | LncRNA small NF90-associated RNA | LCSS | UP | Correlates with cell proliferation, migration and invasion. Its action is related to TGF-ß1 | - | [76] |
| SNHG1 | LncRNA small nucleolar RNA host gene 1 | LSCC | UP | Promotes cell proliferation, migration and invasion. Moreover, induces cell apoptosis and promotes YAPI expression and Hippo signaling activity by miR-375 | Diagnostic biomarker | [77] |
| TUG1 | LncRNA taurine upregulated gene1 | LSCC | UP | Associates with lymph node metastasis. TUG1 silencing inhibits proliferation, cell-cycle progression, migration and invasion | Prognostic biomarker | [78] |
| UCA1 | Urothelial carcinoma- associated 1 | LSCC | UP | Promotes proliferation, migration and invasion by activating Wnt/β-catenin signaling pathway | Diagnostic biomarker | [79] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossu, A.M.; Mosca, L.; Zappavigna, S.; Misso, G.; Bocchetti, M.; De Micco, F.; Quagliuolo, L.; Porcelli, M.; Caraglia, M.; Boccellino, M. Long Non-coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours. Int. J. Mol. Sci. 2019, 20, 3444. https://doi.org/10.3390/ijms20143444
Cossu AM, Mosca L, Zappavigna S, Misso G, Bocchetti M, De Micco F, Quagliuolo L, Porcelli M, Caraglia M, Boccellino M. Long Non-coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours. International Journal of Molecular Sciences. 2019; 20(14):3444. https://doi.org/10.3390/ijms20143444
Chicago/Turabian StyleCossu, Alessia Maria, Laura Mosca, Silvia Zappavigna, Gabriella Misso, Marco Bocchetti, Federica De Micco, Lucio Quagliuolo, Marina Porcelli, Michele Caraglia, and Mariarosaria Boccellino. 2019. "Long Non-coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours" International Journal of Molecular Sciences 20, no. 14: 3444. https://doi.org/10.3390/ijms20143444