Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases
Abstract
:1. MMP Domain Structure and Classification
2. MMP Substrates and Function
3. Multilayered Regulation of MMP Activity
4. PTMs—An Additional Level of Protein Regulation
4.1. Glycosylation of MMP
4.1.1. MMP9
4.1.2. MMP14
4.1.3. MMP1
4.1.4. MMP2
4.1.5. MMP3
4.1.6. MMP13
4.1.7. MMP17
4.2. Phosphorylation of MMPs
4.2.1. MMP2
4.2.2. MMP14
4.2.3. Extracellular Phosphorylation of MMPs
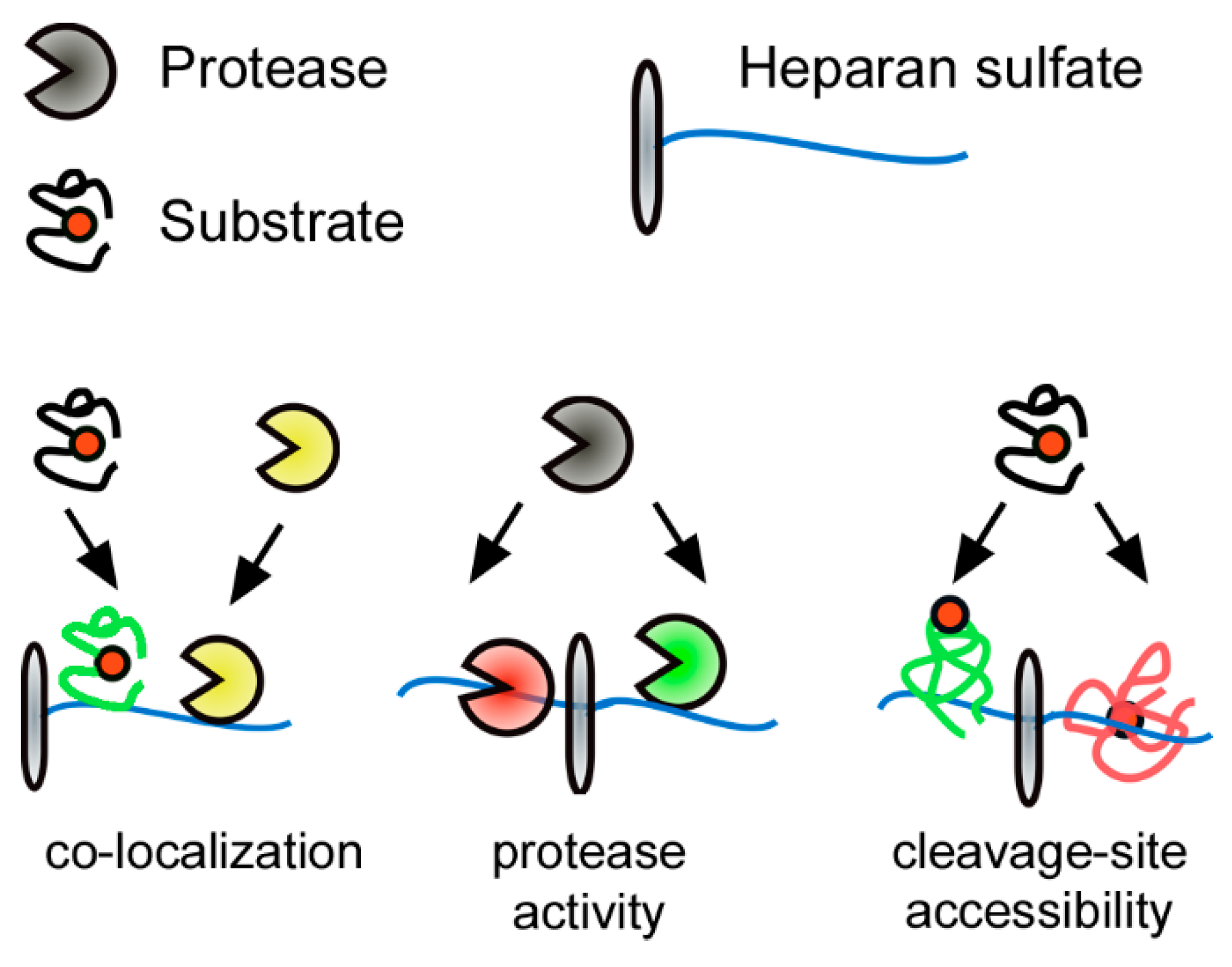
4.3. Glycosaminoglycans
4.3.1. GAG-regulated Substrate Proteolysis
4.3.2. GAG-regulated MMP Activity
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Asn | Asparagine amino acid |
| CA | Cysteine array |
| Cy | Cytosolic domain |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| ER | Endoplasmic reticulum |
| Fu | Furin-recognition site |
| GalNAc | N-acetyl galactosamine |
| GlcNAc | N-acetyl glucosamine |
| GPI | Glycosylphosphatidylinositol |
| LRP | Low-density lipoprotein receptor-related protein |
| MMP | Matrix metalloproteinase |
| Pro | Pro-domain |
| PTMs | Post-translational modifications |
| SA | Signal anchor |
| Ser | Serine amino acid |
| SH | Thiol group |
| SP | Signal peptide |
| Thr | Threonine amino acid |
| TIMPs | Tissue inhibitors of metalloproteinases |
| TM | Transmembrane domain |
| Tyr | Tyrosine amino acid |
| UDP | Uridine diphosphate |
References
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S.; Schilling, O.; Franzke, C.-W.; Claustrat, B.; Brun, J.; Chazot, G. Biological role of matrix metalloproteinases: a critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.J.; Bellac, C.L.; Moraes, T.J.; Wadsworth, S.J.; Dufour, A.; Butler, G.S.; Bilawchuk, L.M.; Hendry, R.G.; Robertson, A.G.; Cheung, C.T.; et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat. Med. 2014, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Cauwe, B.; Opdenakker, G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 351–423. [Google Scholar] [CrossRef]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 20–28. [Google Scholar] [CrossRef]
- Rodríguez, D.; Morrison, C.J.; Overall, C.M. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Murphy, G.; Troeberg, L. Extracellular regulation of metalloproteinases. Matrix Biol. 2015, 44–46, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Huwiler, A.; Akool, E.S.; Aschrafi, A.; Hamada, F.M.A.; Pfeilschifter, J.; Eberhardt, W. ATP Potentiates Interleukin-1??-induced MMP-9 Expression in Mesangial Cells via Recruitment of the ELAV Protein HuR. J. Biol. Chem. 2003, 278, 51758–51769. [Google Scholar] [CrossRef] [PubMed]
- Fähling, M.; Steege, A.; Perlewitz, A.; Nafz, B.; Mrowka, R.; Persson, P.B.; Thiele, B.J. Role of nucleolin in posttranscriptional control of MMP-9 expression. Biochim. Biophys. Acta Gene Struct. Expr. 2005, 1731, 32–40. [Google Scholar] [CrossRef]
- Clark, I.M.; Swingler, T.E.; Sampieri, C.L.; Edwards, D.R. The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 2008, 40, 1362–1378. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Zhang, Z.; Chen, W.; Huang, G.; He, A.; Hou, C.; Long, Y.; Yang, Z.; Zhang, Z.; Liao, W. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1β-induced chondrocyte responses. Osteoarthr. Cartil. 2016, 24, 932–941. [Google Scholar] [CrossRef]
- Bannikov, G.A.; Karelina, T.V.; Collier, I.E.; Marmer, B.L.; Goldberg, G.I. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J. Biol. Chem. 2002, 277, 16022–16027. [Google Scholar] [CrossRef]
- Emonard, H.; Hornebeck, W. Binding of 92 kDa and 72 kDa progelatinases to insoluble elastin modulates their proteolytic activation. Biol. Chem. 1997, 378, 265–271. [Google Scholar] [CrossRef]
- Ra, H.J.; Harju-Baker, S.; Zhang, F.; Linhardt, R.J.; Wilson, C.L.; Parks, W.C. Control of promatrilysin (MMP7) activation and substrate-specific activity by sulfated glycosaminoglycans. J. Biol. Chem. 2009, 284, 27924–27932. [Google Scholar] [CrossRef]
- Geurts, N.; Martens, E.; Van Aelst, I.; Proost, P.; Opdenakker, G.; Van Den Steen, P.E. β-hematin interaction with the hemopexin domain of gelatinase B/MMP-9 provokes autocatalytic processing of the propeptide, thereby priming activation by MMP-3. Biochemistry 2008, 47, 2689–2699. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2009, 29, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.H. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 2002, 115, 3719–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, J.W.; Bennett, E.J. Proteome complexity and the forces that drive proteome imbalance. Nature 2016, 537, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.L.; Park, K.M.; Murray, J.; Kim, K.; Ryu, S.H. Direct Profiling the Post-Translational Modification Codes of a Single Protein Immobilized on a Surface Using Cu-free Click Chemistry. ACS Cent. Sci. 2018, 4, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Deribe, Y.L.; Pawson, T.; Dikic, I. Post-translational modifications in signal integration. Nat. Struct. Mol. Biol. 2010, 17, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Peterson, S.E.; Loring, J.F. Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res. 2014, 24, 143–160. [Google Scholar] [CrossRef]
- Ricard-Blum, S. Protein–Glycosaminoglycan interaction networks: Focus on heparan sulfate. Perspect. Sci. 2017. [Google Scholar] [CrossRef]
- Audagnotto, M.; Dal Peraro, M. Protein post-translational modifications: In silico prediction tools and molecular modeling. Comput. Struct. Biotechnol. J. 2017, 15, 307–319. [Google Scholar] [CrossRef]
- Spoel, S.H. Orchestrating the proteome with post-translational modifications. J. Exp. Bot. 2018, 69, 4499–4503. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.D.; Overall, C.M. Proteolytic Post-translational Modification of Proteins: Proteomic Tools and Methodology. Mol. Cell. Proteomics 2013, 12, 3532–3542. [Google Scholar] [CrossRef] [Green Version]
- Beltrao, P.; Bork, P.; Krogan, N.J.; van Noort, V.; Abu-Qarn, M.; Eichler, J.; Sharon, N.; Alexander, J.; Lim, D.; Joughin, B.; et al. Evolution and functional cross-talk of protein post-translational modifications. Mol. Syst. Biol. 2013, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, K.J. Post-translational modifications and their biological functions: proteomic analysis and systematic approaches. J. Biochem. Mol. Biol. 2004, 37, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Vanheule, V.; Metzemaekers, M.; Janssens, R.; Struyf, S.; Proost, P. How post-translational modifications influence the biological activity of chemokines. Cytokine 2018, 109, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, F. Post-translational modification control of HBV biological processes. Front. Microbiol. 2018, 9, 2661. [Google Scholar] [CrossRef] [PubMed]
- Arbez, N.; Ratovitski, T.; Roby, E.; Chighladze, E.; Stewart, J.C.; Ren, M.; Wang, X.; Lavery, D.J.; Ross, C.A. Post-translational modifications clustering within proteolytic domains decrease mutant huntingtin toxicity. J. Biol. Chem. 2017, 292, 19238–19249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, B.J.; Nissim, A.; Winyard, P.G. Oxidative post-translational modifications and their involvement in the pathogenesis of autoimmune diseases. Redox Biol. 2014, 2, 715–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeken, J. Glycosylation and its Disorders: General Overview. Ref. Modul. Biomed. Sci. 2016, 1–8. [Google Scholar] [CrossRef]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef]
- Boon, L.; Ugarte-Berzal, E.; Vandooren, J.; Opdenakker, G. Glycosylation of matrix metalloproteases and tissue inhibitors: present state, challenges and opportunities. Biochem. J. 2016, 473, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Aebi, M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 2011, 21, 576–582. [Google Scholar] [CrossRef]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.B.G.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiro RG Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43R–56R. [CrossRef] [PubMed]
- Tian, E.; Ten Hagen, K.G. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj. J. 2009, 26, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.J.; Clausen, H.; Bard, F. Location, location, location: New insights into O-GalNAc protein glycosylation. Trends Cell Biol. 2011, 21, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Guerrero, R. Recent structural and mechanistic insights into protein O-GalNAc glycosylation. Biochem. Soc. Trans. 2016, 44, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Aebi, M. Intracellular functions of N-linked glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef]
- Lee, H.S.; Qi, Y.; Im, W. Effects of N-glycosylation on protein conformation and dynamics: Protein Data Bank analysis and molecular dynamics simulation study. Sci. Rep. 2015, 5, 8926. [Google Scholar] [CrossRef] [PubMed]
- Goettig, P. Effects of glycosylation on the enzymatic activity and mechanisms of proteases. Int. J. Mol. Sci. 2016, 17, 1969. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.; Fu, J.; Xia, L. Biological Functions of C1GalT1 and Mucin-Type O-Glycans. In Glycoscience: Biology and Medicine; Springer: Tokyo, Japan, 2015; pp. 1073–1080. ISBN 9784431548416. [Google Scholar]
- Tran, D.T.; Ten Hagen, K.G. Mucin-type o-glycosylation during development. J. Biol. Chem. 2013, 288, 6921–6929. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Hincapie, M.; Packer, N.; Baker, M.S.; Hancock, W.S.; Fanayan, S. An optimized approach for enrichment of glycoproteins from cell culture lysates using native multi-lectin affinity chromatography. J. Sep. Sci. 2012, 35, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van Den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Kotra, L.P.; Zhang, L.; Fridman, R.; Orlando, R.; Mobashery, S. N-glycosylation pattern of the zymogenic form of human matrix metalloproteinase-9. Bioorg. Chem. 2002, 30, 356–370. [Google Scholar] [CrossRef]
- Roth, J. Protein N-glycosylation along the Secretory Pathway: Relationship to organelle topography and function, protein quality control, and cell interactions. Chem. Rev. 2002, 102, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Duellman, T.; Burnett, J.; Yang, J. Functional Roles of N-Linked Glycosylation of Human Matrix Metalloproteinase 9. Traffic 2015, 16, 1108–1126. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Cieplak, P. Role of N-glycosylation in activation of proMMP-9. A molecular dynamics simulations study. PLoS ONE 2018, 13, e0191157. [Google Scholar] [CrossRef] [PubMed]
- Nishi, N.; Shoji, H.; Seki, M.; Itoh, A.; Miyanaka, H.; Yuube, K.; Hirashima, M.; Nakamura, T. Galectin-8 modulates neutrophil function via interaction with integrin αM. Glycobiology 2003, 13, 755–763. [Google Scholar] [CrossRef]
- Boon, L.; Ugarte-Berzal, E.; Martens, E.; Vandooren, J.; Rybakin, V.; Colau, D.; Gordon-Alonso, M.; van der Bruggen, P.; Stöcker, W.; Becker-Pauly, C.; et al. Propeptide glycosylation and galectin-3 binding decrease proteolytic activation of human proMMP-9/progelatinase B. FEBS J. 2019, 285, 930–945. [Google Scholar] [CrossRef]
- Van Den Steen, P.E.; Van Aelst, I.; Hvidberg, V.; Piccard, H.; Fiten, P.; Jacobsen, C.; Moestrup, S.K.; Fry, S.; Royle, L.; Wormald, M.R.; et al. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J. Biol. Chem. 2006, 281, 18626–18637. [Google Scholar] [CrossRef]
- Mattu, T.S.; Royle, L.; Langridge, J.; Wormald, M.R.; Van den Steen, P.E.; Van Damme, J.; Opdenakker, G.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M. O-glycan analysis of natural human neutrophil gelatinase B using a combination of normal phase- HPLC and online tandem mass spectrometry: Implications for the domain organization of the enzyme. Biochemistry 2000, 39, 15695–15704. [Google Scholar] [CrossRef]
- Rosenblum, G.; Van den Steen, P.E.; Cohen, S.R.; Grossmann, J.G.; Frenkel, J.; Sertchook, R.; Slack, N.; Strange, R.W.; Opdenakker, G.; Sagi, I. Insights into the Structure and Domain Flexibility of Full-Length Pro-Matrix Metalloproteinase-9/Gelatinase B. Structure 2007, 15, 1227–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandooren, J.; Knoops, S.; Buzzo, J.L.A.; Boon, L.; Martens, E.; Opdenakker, G.; Kolaczkowska, E. Differential inhibition of activity, activation and gene expression of MMP-9 in THP-1 cells by azithromycin and minocycline versus bortezomib: A comparative study. PLoS ONE 2017, 12, e0174853. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J. Gelatin degradation assay reveals MMP-9 inhibitors and function of O-glycosylated domain. World J. Biol. Chem. 2011, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.; Drews, M.; Conner, C.; Foda, H.D.; DeClerck, Y.A.; Langley, K.E.; Bahou, W.F.; Docherty, A.J.P.; Cao, J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP). J. Biol. Chem. 1998, 273, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Sato, H.; Okada, A.; Ohuchi, E.; Imai, K.; Okada, Y.; Seiki, M. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J. Biol. Chem. 1998, 273, 16098–16103. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.I.; Munshi, H.G.; Sen, R.; Snipas, S.J.; Salvesen, G.S.; Fridman, R.; Stack, M.S. Glycosylation Broadens the Substrate Profile of Membrane Type 1 Matrix Metalloproteinase. J. Biol. Chem. 2004, 279, 8278–8289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Growth, T.; Nguyen, A.T.; Chia, J.; Ros, M.; Hui, K.M.; Saltel, F.; Bard, F. Organelle Specific O-Glycosylation Drives MMP14 Activation, Tumor Growth, and Metastasis. Cancer Cell 2017, 32, 639–653.e6. [Google Scholar] [CrossRef] [Green Version]
- Saarinen, J.; Welgus, H.G.; Flizar, C.A.; Kalkkinen, N.; Helin, J. N-Glycan structures of matrix metalloproteinase-1 derived from human fibroblasts and from HT-1080 fibrosarcoma cells. Eur. J. Biochem. 1999, 259, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Piccard, H.; Van den Steen, P.E.; Opdenakker, G. Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J. Leukoc. Biol. 2007, 81, 870–892. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef] [Green Version]
- García-Pardo, A.; Opdenakker, G. Nonproteolytic functions of matrix metalloproteinases in pathology and insights for the development of novel therapeutic inhibitors. Met. Med. 2015, 2, 19–28. [Google Scholar] [CrossRef]
- Nagase, H. Matrix Metalloproteinase 3/Stromelysin 1. In Handbook of Proteolytic Enzymes, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 763–774. [Google Scholar] [CrossRef]
- Henriet, P.; Eeckhout, Y. Eeckhout, Y. Matrix Metallopeptidase-13/Collagenase 3. In Handbook of Proteolytic Enzymes, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 734–744. [Google Scholar] [CrossRef]
- Knäuper, V.; López-Otin, C.; Smith, B.; Knight, G.; Murphy, G. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996, 271, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Hieronimus, B.; Pfohl, J.; Busch, C.; Graeve, L. Expression and characterization of membrane-type 4 matrix metalloproteinase (MT4-MMP) and its different forms in melanoma. Cell. Physiol. Biochem. 2017, 42, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Kajita, M.; Kinoh, H.; Mori, H.; Okada, A.; Seiki, M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J. Biol. Chem. 1999, 274, 34260–34266. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Marco, M.; Zhao, H.; Shi, Q.; Merriman, S.; Mobashery, S.; Fridman, R. Characterization of the dimerization interface of membrane type 4 (MT4)-matrix metalloproteinase. J. Biol. Chem. 2011, 286, 33178–33189. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L. Lo The crucial role of protein phosphorylation in cell signalingand its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Nishi, H.; Shaytan, A.; Panchenko, A.R. Physicochemical mechanisms of protein regulation by phosphorylation. Front. Genet. 2014, 5, 270. [Google Scholar] [CrossRef] [Green Version]
- Cutillas, P.R. Targeted In-Depth Quantification of Signaling Using Label-Free Mass Spectrometry. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 585, pp. 245–268. ISBN 9780128097427. [Google Scholar]
- Cohen, P. The role of protein phosphorylation in human health and disease. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef]
- Sariahmetoglu, M.; Crawford, B.D.; Leon, H.; Sawicka, J.; Li, L.; Ballermann, B.J.; Holmes, C.; Berthiaume, L.G.; Holt, A.; Sawicki, G.; et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007, 21, 2486–2495. [Google Scholar] [CrossRef]
- Jacob-Ferreira, A.L.; Kondo, M.Y.; Baral, P.K.; James, M.N.G.; Holt, A.; Fan, X.; Schulz, R. Phosphorylation Status of 72 kDa MMP-2 Determines Its Structure and Activity in Response to Peroxynitrite. PLoS ONE 2013, 8, e71794. [Google Scholar] [CrossRef]
- 3rd Millenium, PhosphoSitePlus. Cell Signalling Technology Inc. 2011. Available online: http://www.phosphosite.org/proteinAction.do?id=662&showAllSites=true (accessed on 22 May 2019).
- UniProt Consortium, T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2018, 46, 2699. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.C.; Coppolino, M.G. Phosphorylation of membrane type 1-matrix metalloproteinase (MT1-MMP) and its vesicle-associated membrane protein 7 (VAMP7)-dependent trafficking facilitate cell invasion and migration. J. Biol. Chem. 2011, 286, 43405–43416. [Google Scholar] [CrossRef] [PubMed]
- Moss, N.M.; Wu, Y.I.; Liu, Y.; Munshi, H.G.; Stack, M.S. Modulation of the membrane type 1 matrix metalloproteinase cytoplasmic tail enhances tumor cell invasion and proliferation in three-dimensional collagen matrices. J. Biol. Chem. 2009, 284, 19791–19799. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kasberg, W.C.; Celo, A.; Liang, Z.; Quispe, K.; Sharon Stack, M. Post-translational modification of the membrane type 1 matrix metalloproteinase (MT1-MMP) cytoplasmic tail impacts ovarian cancer multicellular aggregate dynamics. J. Biol. Chem. 2017, 292, 13111–13121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyalendo, C.; Michaud, M.; Beaulieu, E.; Roghi, C.; Murphy, G.; Gingras, D.; Béliveau, R. Src-dependent Phosphorylation of Membrane Type I Matrix Metalloproteinase on Cytoplasmic Tyrosine 573. J. Biol. Chem. 2007, 282, 15690–15699. [Google Scholar] [CrossRef] [Green Version]
- Moss, N.M.; Liu, Y.; Johnson, J.J.; Debiase, P.; Jones, J.; Hudson, L.G.; Munshi, H.G.; Stack, M.S. Epidermal Growth Factor Receptor-Mediated Membrane Type 1 Matrix Metalloproteinase Endocytosis Regulates the Transition between Invasive versus Expansive Growth of Ovarian Carcinoma Cells in Three-Dimensional Collagen. Mol. Cancer Res. 2009, 7, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Rinschen, M.M.; Yu, M.-J.; Wang, G.; Boja, E.S.; Hoffert, J.D.; Pisitkun, T.; Knepper, M.A. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor–dependent signaling pathways in renal collecting duct cells. Proc. Natl. Acad. Sci. USA 2010, 107, 3882–3887. [Google Scholar] [CrossRef]
- Bordoli, M.R.; Yum, J.; Breitkopf, S.B.; Thon, J.N.; Italiano, J.E.; Xiao, J.; Worby, C.; Wong, S.K.; Lin, G.; Edenius, M.; et al. A secreted tyrosine kinase acts in the extracellular environment. Cell 2014, 158, 1033–1044. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Wiley, S.E.; Guo, X.; Kinch, L.N.; Durrant, E.; Wen, J.; Xiao, J.; Cui, J.; Nguyen, K.B.; Engel, J.L.; et al. A Single Kinase Generates the Majority of the Secreted Phosphoproteome. Cell 2015, 161, 1619–1632. [Google Scholar] [CrossRef] [Green Version]
- Sreelatha, A.; Kinch, L.N.; Tagliabracci, V.S. The secretory pathway kinases. Biochim. Biophys. Acta Proteins Proteomics 2015, 1854, 1687–1693. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, H.O.; Takeuchi, H.; Haltiwanger, R.S.; Irvine, K.D. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science 2008, 321, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004, 131, 6009–6021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proudfoot, E.A.; Johnson, Z.; Bonvin, P.; Handel, M.T. Glycosaminoglycan Interactions with Chemokines Add Complexity to a Complex System. Pharmaceuticals 2017, 10, 70. [Google Scholar] [CrossRef]
- Raman, R.; Sasisekharan, V.; Sasisekharan, R. Structural Insights into Biological Roles of Protein-Glycosaminoglycan Interactions. Chem. Biol. 2005, 12, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Esko, J.D. Demystifying Heparan Sulfate–Protein Interactions. Annu. Rev. Biochem. 2014, 83, 129–157. [Google Scholar] [CrossRef]
- Lin, X.; Buff, E.M.; Perrimon, N.; Michelson, A.M. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development 1999, 126, 3715–3723. [Google Scholar] [PubMed]
- Guimond, S.; Maccarana, M.; Olwin, B.B.; Lindahl, U.; Rapraeger, A.C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J. Biol. Chem. 1993, 268, 23906–23914. [Google Scholar]
- Pye, D.A.; Vives, R.R.; Turnbull, J.E.; Hyde, P.; Gallagher, J.T. Heparan Sulfate Oligosaccharides Require 6-O-Sulfation for Promotion of Basic Fibroblast Growth Factor Mitogenic Activity. J. Biol. Chem. 1998, 273, 22936–22942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapraeger, A.C. In the clutches of proteoglycans: how does heparan sulfate regulate FGF binding? Chem. Biol. 1995, 2, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Lee, S.; Wang, Y.; Ha, Y. Crystal Structure of the E2 Domain of Amyloid Precursor Protein-like Protein 1 in Complex with Sucrose Octasulfate. J. Biol. Chem. 2011, 286, 29748–29757. [Google Scholar] [CrossRef] [Green Version]
- Gralle, M.; Botelho, M.G.; Wouters, F.S. Neuroprotective Secreted Amyloid Precursor Protein Acts by Disrupting Amyloid Precursor Protein Dimers. J. Biol. Chem. 2009, 284, 15016–15025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogewerf, A.J.; Kuschert, G.S.V.; Proudfoot, A.E.I.; Borlat, F.; Clark-Lewis, I.; Power, C.A.; Wells, T.N.C. Glycosaminoglycans Mediate Cell Surface Oligomerization of Chemokines. Biochemistry 1997, 36, 13570–13578. [Google Scholar] [CrossRef] [PubMed]
- Salanga, C.L.; Handel, T.M. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: The role of structural dynamics in function. Exp. Cell Res. 2011, 317, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Gospodarowicz, D.; Cheng, J. Heparin protects basic and acidic FGF from inactivation. J. Cell. Physiol. 1986, 128, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Saksela, O.; Moscatelli, D.; Sommer, A.; Rifkin, D.B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J. Cell Biol. 1988, 107, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Sadir, R.; Imberty, A.; Baleux, F.; Lortat-Jacob, H. Heparan Sulfate/Heparin Oligosaccharides Protect Stromal Cell-derived Factor-1 (SDF-1)/CXCL12 against Proteolysis Induced by CD26/Dipeptidyl Peptidase IV. J. Biol. Chem. 2004, 279, 43854–43860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lortat-Jacob, H.; Baltzer, F.; Grimaud, J.-A. Heparin Decreases the Blood Clearance of Interferon-$γ$ and Increases Its Activity by Limiting the Processing of Its Carboxyl-terminal Sequence. J. Biol. Chem. 1996, 271, 16139–16143. [Google Scholar] [CrossRef]
- Jakobs, P.; Schulz, P.; Ortmann, C.; Schürmann, S.; Exner, S.; Rebollido-Rios, R.; Dreier, R.; Seidler, D.G.; Grobe, K. Bridging the gap: heparan sulfate and Scube2 assemble Sonic hedgehog release complexes at the surface of producing cells. Sci. Rep. 2016, 6, 26435. [Google Scholar] [CrossRef]
- Jakobs, P.; Schulz, P.; Schürmann, S.; Niland, S.; Exner, S.; Rebollido-Rios, R.; Manikowski, D.; Hoffmann, D.; Seidler, D.G.; Grobe, K. Ca2+ coordination controls sonic hedgehog structure and its Scube2-regulated release. J. Cell Sci. 2017, 130, 3261–3271. [Google Scholar] [CrossRef]
- Kastl, P.; Manikowski, D.; Steffes, G.; Schürmann, S.; Bandari, S.; Klämbt, C.; Grobe, K. Disrupting Hedgehog Cardin-Weintraub sequence and positioning changes cellular differentiation and compartmentalization in vivo. Development 2018, 145, dev167221. [Google Scholar] [CrossRef]
- Cerqueira, C.; Samperio Ventayol, P.; Vogeley, C.; Schelhaas, M. Kallikrein-8 Proteolytically Processes Human Papillomaviruses in the Extracellular Space To Facilitate Entry into Host Cells. J. Virol. 2015, 89, 7038–7052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, Y.; Naruo, H.; Yoshitomi, Y.; Munesue, S.; Kiyono, S.; Kusano, Y.; Hashimoto, K.; Yokoi, T.; Nakanishi, H.; Shimizu, S.; et al. Matrix Metalloproteinase-9 Associated with Heparan Sulphate Chains of GPI-Anchored Cell Surface Proteoglycans Mediates Motility of Murine Colon Adenocarcinoma Cells. J. Biochem. 2008, 143, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-H.; Woessner, J.F. Heparan Sulfate Proteoglycans as Extracellular Docking Molecules for Matrilysin (Matrix Metalloproteinase 7). J. Biol. Chem. 2000, 275, 4183–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, G.S.; Apte, S.S.; Willenbrock, F.; Murphy, G. Human Tissue Inhibitor of Metalloproteinases 3 Interacts with Both the N- and C-terminal Domains of Gelatinases A and B: REGULATION BY POLYANIONS. J. Biol. Chem. 1999, 274, 10846–10851. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Jung, K.; Tonti, G.A.; Canestrari, F. Heparin affects matrix metalloproteinases and tissue inhibitors of metalloproteinases circulating in peripheral blood. Clin. Biochem. 2008, 41, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Di Simone, N.; Di Nicuolo, F.; Sanguinetti, M.; Ferrazzani, S.; D’Alessio, M.C.; Castellani, R.; Bompiani, A.; Caruso, A. Low-molecular Weight Heparin Induces In Vitro Trophoblast Invasiveness: Role of Matrix Metalloproteinases and Tissue Inhibitors. Placenta 2007, 28, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Isnard, N.; Robert, L.; Renard, G. Effect of Sulfated GAGs on the Expression and Activation of MMP-2 and MMP-9 in Corneal and Dermal Explant Cultures. Cell Biol. Int. 2003, 27, 779–784. [Google Scholar] [CrossRef]
- Rababah, M.; Worthmann, H.; Deb, M.; Tryc, A.B.; Ma, Y.T.; El Bendary, O.M.; Hecker, H.; Goldbecker, A.; Heeren, M.; Brand, K.; et al. Anticoagulants affect matrix metalloproteinase 9 levels in blood samples of stroke patients and healthy controls. Clin. Biochem. 2012, 45, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, E.; Pejler, G.; Ringvall, M.; Lunderius, C.; Tomasini-Johansson, B.; Kusche-Gullberg, M.; Eriksson, I.; Ledin, J.; Hellman, L.; Kjellén, L. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 1999, 400, 773. [Google Scholar] [CrossRef] [PubMed]
- Humphries, D.E.; Wong, G.W.; Friend, D.S.; Gurish, M.F.; Qiu, W.-T.; Huang, C.; Sharpe, A.H.; Stevens, R.L. Heparin is essential for the storage of specific granule proteases in mast cells. Nature 1999, 400, 769. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Mann, M. Status of Large-scale Analysis of Post-translational Modifications by Mass Spectrometry. Mol. Cell. Proteomics 2013, 12, 3444–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryšlavá, H.; Doubnerová, V.; Kavan, D.; Vaněk, O. Effect of posttranslational modifications on enzyme function and assembly. J. Proteomics 2013, 92, 80–109. [Google Scholar] [CrossRef] [PubMed]
- Pascovici, D.; Wu, J.X.; McKay, M.J.; Joseph, C.; Noor, Z.; Kamath, K.; Wu, Y.; Ranganathan, S.; Gupta, V.; Mirzaei, M. Clinically Relevant Post-Translational Modification Analyses-Maturing Workflows and Bioinformatics Tools. Int. J. Mol. Sci. 2018, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Eisen, A.Z.; Teter, M.; Clark, S.D.; Kronberger, A.; Goldberg, G. Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc. Natl. Acad. Sci. USA 2006, 83, 3756–3760. [Google Scholar] [CrossRef] [PubMed]
- Huanna, T.; Tao, Z.; Xiangfei, W.; Longfei, A.; Yuanyuan, X.; Jianhua, W.; Cuifang, Z.; Manjing, J.; Wenjing, C.; Shaochuan, Q.; et al. GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol. Carcinog. 2015, 54, 1159–1171. [Google Scholar] [CrossRef] [PubMed]

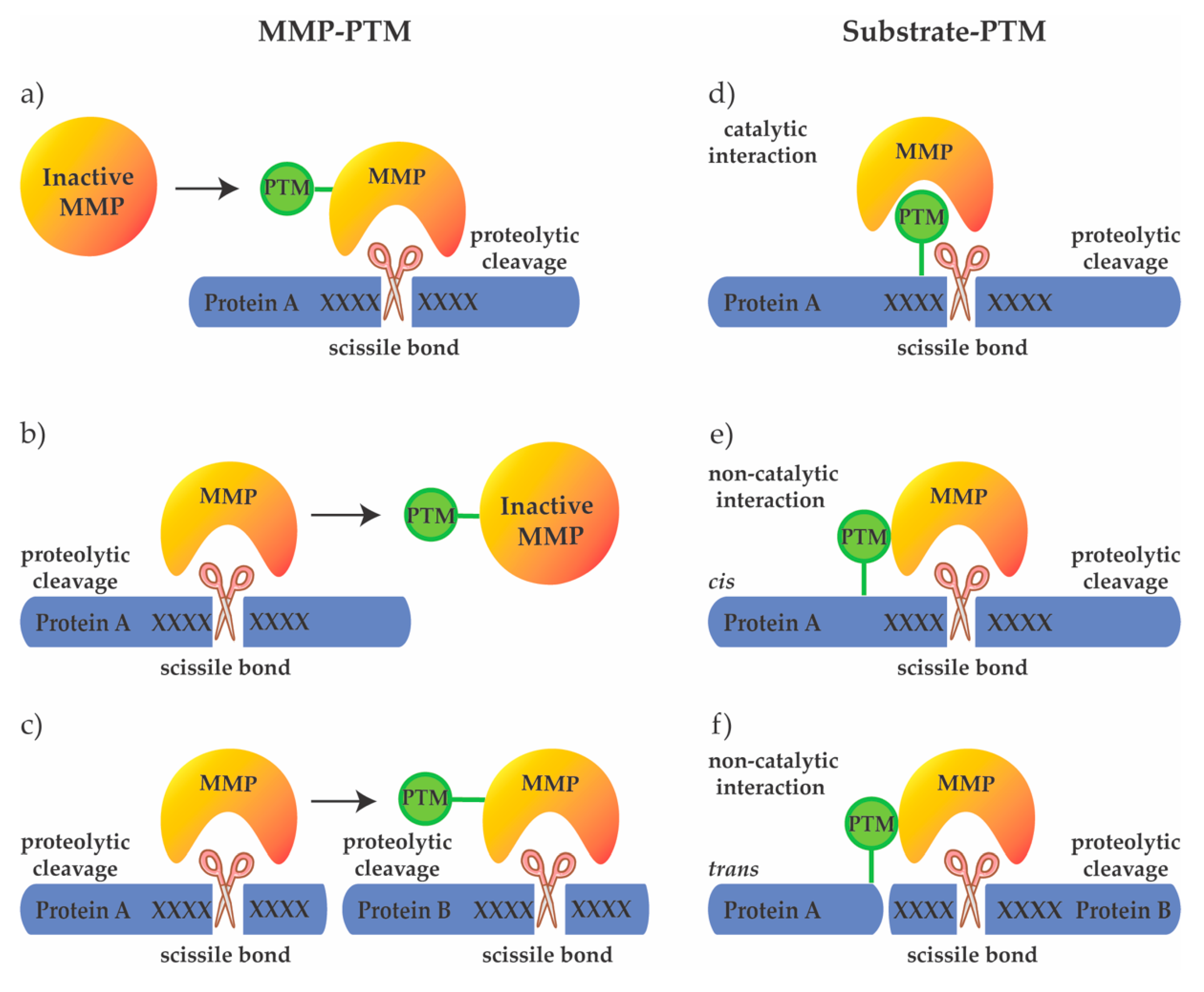
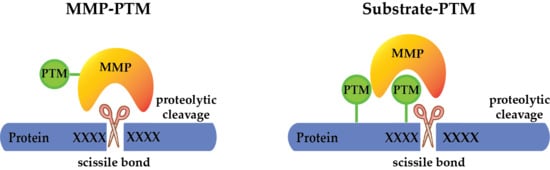
| MMP | Modification | Biological Effect | Reference |
|---|---|---|---|
| MMP1 | N-linked glycosylation at Asn120 | Tumor cell invasion and angiogenesis | [39,69,129] |
| Phosphorylation at Tyr360 | Not reported | [93] | |
| MMP2 | O-linked glycosylation at Ser32, Thr96, 262, 458, 460 | Upregulation of MMP2 | [39,71,72] |
| N-linked glycosylation at Asn573 and Asn642 | Not reported | [130] | |
| Phosphorylation at Ser32, Ser160, Tyr271, Thr250 and Ser365 | Phosphorylation decreases, while dephosphorylation increases protease activity | [83,84] | |
| Heparan sulfate | Cell surface localization; affects protease activity by increasing TIMP3 affinity | [119,120,121,122] | |
| MMP3 | N-linked glycosylation at Asn120 and Asn398 | Not reported | [39,73] |
| Three potential O-linked glycosylation at Ser56, Ser269 and Thr277 | |||
| MMP7 | Heparan sulfate, Chondroitin sulfate | Cell surface localization; affects protease activity by increasing TIMP3 affinity; increases MMP7 auto-processing and activity | [19,120,121] |
| MMP9 | N-linked glycosylation at Asn38 and Asn120 | MMP9 secretion and activation | [53,54,55,56,57,58,59] |
| O-linked glycosylation in the linker region | Increases the domain flexibility; necessary for internalization and degradation; protects against proteolytic degradation; reduces gelatinolytic activity | [60,62,63,64] | |
| Heparan sulfate | Cell surface localization; affects protease activity by increasing TIMP3 affinity; affects MMP9 expression and plasma levels | [119,120,121,122,123] | |
| MMP12 | Phosphorylation at Tyr414 | Not reported | [93] |
| MMP13 | N-linked glycosylation at Asn117 and Asn152 | Not reported | [1,74,75] |
| O-linked glycosylation at Ser24 and Ser62 | Not reported | [1,74,75] | |
| Phosphorylation at Tyr366 | Not reported | [93] | |
| MMP14 | N-linked glycosylation at Asn229 and Asn311 | Not reported | [39] |
| O-linked glycosylation at Thr291, Thr299, Thr300, and Ser301 | Required for formation of a stable complex with proMMP2 and TIMP2; increases activity upon glycosylation perturbation | [65,66,67,68] | |
| Phosphorylation at Thr567, Tyr573 and Tyr353 | Regulates MMP14 induced cellular invasion and migration; cell surface dynamics and internalization; mimetic mutants exhibit higher collagenolytic activity and three-dimensional growth; promotes metastasis-associated behaviors | [72,87,88,89,90,91,93] | |
| MMP16 | Phosphorylation at Tyr377 and Tyr521 | Not reported | [93] |
| MMP17 | N-linked glycosylation at Asn137 and Asn318 | Stabilizes the dimeric form of MMP17 | [76,77,78] |
| MMP24 | Phosphorylation at Tyr534 | Not reported | [93] |
| MMP27 | Phosphorylation at Tyr360 | Not reported | [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madzharova, E.; Kastl, P.; Sabino, F.; auf dem Keller, U. Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases. Int. J. Mol. Sci. 2019, 20, 3077. https://doi.org/10.3390/ijms20123077
Madzharova E, Kastl P, Sabino F, auf dem Keller U. Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases. International Journal of Molecular Sciences. 2019; 20(12):3077. https://doi.org/10.3390/ijms20123077
Chicago/Turabian StyleMadzharova, Elizabeta, Philipp Kastl, Fabio Sabino, and Ulrich auf dem Keller. 2019. "Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases" International Journal of Molecular Sciences 20, no. 12: 3077. https://doi.org/10.3390/ijms20123077