Comparative Study of the Molecular Basis of Pathogenicity of M. bovis Strains in a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mice and M. bovis Strains
2.3. Genomic DNA Extraction, Sequencing, and Analysis
2.4. Bacterial Infection
2.5. Histopathology
2.6. Multiplex Cytokine Assay
2.7. Statistical Analysis
3. Results
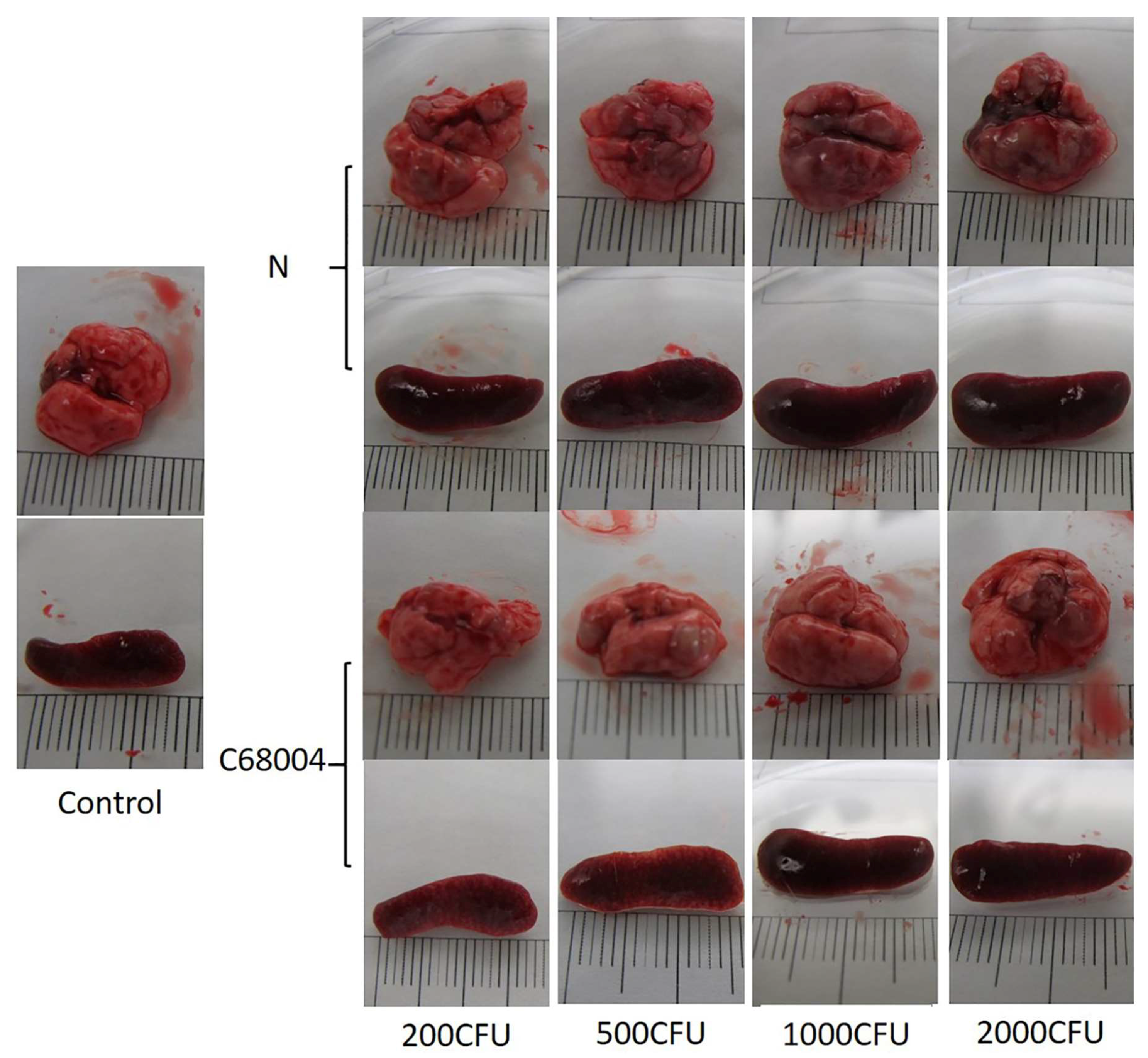
3.1. Clinical Presentation and Necropsy Lesions
3.2. Survival Time
3.3. Organ Coefficient and Clinical Score of Mice Infected with Different Strains of M. bovis
3.4. Histopathological Examination
3.5. Cytokine Profiles in Serum
3.6. Genomic Sequence Analysis
3.7. Virulence Factors Mutation in Both Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report. Geneva; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Muller, B.; Durr, S.; Alonso, S.; Hattendorf, J.; Laisse, C.J.; Parsons, S.D.; van Helden, P.D.; Zinsstag, J. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg. Infect. Dis. 2013, 19, 899–908. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, R.; Vallania, F.; Yang, Q.; Lopez Angel, C.J.; Darboe, F.; Penn-Nicholson, A.; Rozot, V.; Nemes, E.; Malherbe, S.T.; Ronacher, K.; et al. A multi-cohort study of the immune factors associated with M. Tuberculosis infection outcomes. Nature 2018, 560, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, L.B.; Tailleux, L.; Pai, A.A.; Gicquel, B.; Marioni, J.C.; Gilad, Y. Deciphering the genetic architecture of variation in the immune response to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 2012, 109, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Wang, X.; Xiao, S.; Li, M.; Li, G.; Zhao, L.; Zhao, X.; Dou, X.; Wan, K. Genetic diversity of immune-related antigens in region of difference 2 of Mycobacterium tuberculosis strains. Tuberculosis 2017, 104, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Portevin, D.; Gagneux, S.; Comas, I.; Young, D. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog. 2011, 7, e1001307. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lv, Y.; Sreevatsan, S.; Zhao, D.; Zhou, X. Differences in pathogenicity of three animal isolates of Mycobacterium species in a mouse model. PLoS ONE 2017, 12, e0183666. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., 3rd; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Jia, X.; Yang, L.; Dong, M.; Chen, S.; Lv, L.; Cao, D.; Fu, J.; Yang, T.; Zhang, J.; Zhang, X.; et al. The bioinformatics analysis of comparative genomics of Mycobacterium tuberculosis complex (MTBC) provides insight into dissimilarities between intraspecific groups differing in host association, virulence, and epitope diversity. Front. Cell. Infect. Microbiol. 2017, 7, 88. [Google Scholar] [CrossRef]
- Garbaccio, S.; Macias, A.; Shimizu, E.; Paolicchi, F.; Pezzone, N.; Magnano, G.; Zapata, L.; Abdala, A.; Tarabla, H.; Peyru, M.; et al. Association between spoligotype-VNTR types and virulence of Mycobacterium bovis in cattle. Virulence 2014, 5, 297–302. [Google Scholar] [CrossRef]
- Peters, J.S.; Calder, B.; Gonnelli, G.; Degroeve, S.; Rajaonarifara, E.; Mulder, N.; Soares, N.C.; Martens, L.; Blackburn, J.M. Identification of quantitative proteomic differences between Mycobacterium tuberculosis lineages with altered virulence. Front. Microbiol. 2016, 7, 813. [Google Scholar] [CrossRef] [PubMed]
- Coscolla, M.; Gagneux, S. Does M. Tuberculosis genomic diversity explain disease diversity? Drug Discov. Today Dis. Mech. 2010, 7, e43–e59. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, S.; Small, P.M. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect. Dis. 2007, 7, 328–337. [Google Scholar] [CrossRef]
- Coscolla, M.; Gagneux, S. Consequences of genomic diversity in mycobacterium tuberculosis. Semin. Immunol. 2014, 26, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.E.; Gavier-Widen, D.; Hewinson, R.G.; Hogarth, P.J. Development of a Mycobacterium bovis intranasal challenge model in mice. Tuberculosis 2008, 88, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Gibson, S.; Garcia-Jimenez, W.; Gough, J.; Strickland, T.S.; Vordermeier, H.M.; Villarreal-Ramos, B. Differential cell composition and cytokine expression within lymph node granulomas from BCG-vaccinated and non-vaccinated cattle experimentally infected with Mycobacterium bovis. Transbound. Emerg. Dis. 2017, 64, 1734–1749. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–697. [Google Scholar] [CrossRef] [PubMed]
- Huard, R.C.; Lazzarini, L.C.; Butler, W.R.; van Soolingen, D.; Ho, J.L. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J. Clin. Microbiol. 2003, 41, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FASTQ data to high confidence variant calls: The genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar]
- Cingolani, P.; Platts, A.; Wang le, L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEFF: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Boute, M.; Carreras, F.; Rossignol, C.; Doz, E.; Winter, N.; Epardaud, M. The C3HeB/FeJ mouse model recapitulates the hallmark of bovine tuberculosis lung lesions following Mycobacterium bovis aerogenous infection. Vet. Res. 2017, 48, 73. [Google Scholar] [CrossRef] [PubMed]
- Kato-Maeda, M.; Shanley, C.A.; Ackart, D.; Jarlsberg, L.G.; Shang, S.; Obregon-Henao, A.; Harton, M.; Basaraba, R.J.; Henao-Tamayo, M.; Barrozo, J.C.; et al. Beijing sublineages of Mycobacterium tuberculosis differ in pathogenicity in the guinea pig. Clin. Vaccine Immunol. CVI 2012, 19, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Dibbern, J.; Eggers, L.; Schneider, B.E. Sex differences in the C57BL/6 model of Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, 10957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; He, Y.; Zhan, X.; Xu, L. IPR1 modified bcg as a novel vaccine induces stronger immunity than bcg against tuberculosis infection in mice. Mol. Med. Rep. 2016, 14, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.X.; Ge, P.P.; Li, J.; Wang, Q.; Gao, G.F.; Qiu, X.B.; Liu, C.H. Mycobacterium tuberculosis suppresses innate immunity by coopting the host ubiquitin system. Nat. Immunol. 2015, 16, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wu, X.; Hu, J.; Hu, M.; Xu, H.; Jiang, H.; Ding, Q. Maternal high-fat diet promotes the development and progression of prostate cancer in transgenic adenocarcinoma mouse prostate offspring. Cell. Physiol. Biochem. 2018, 47, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, K.R.; Duan, L.; Wang, Y.; Hu, W.; Wang, N.; Sun, Q.; Liu, Q.; Liu, X.; Hou, X.; Cheng, A.; et al. Serum cytokines Th1, Th2, and Th17 expression profiling in active lupus nephritis-iv: From a southern chinese han population. Mediat. Inflamm. 2016, 2016, 4927530. [Google Scholar] [CrossRef]
- North, R.J.; Jung, Y.J. Immunity to tuberculosis. Annu. Rev. Immunol. 2004, 22, 599–623. [Google Scholar] [CrossRef]
- Comas, I.; Chakravartti, J.; Small, P.M.; Galagan, J.; Niemann, S.; Kremer, K.; Ernst, J.D.; Gagneux, S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 2010, 42, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wang, R.; Deng, D.; Chen, Y.; Liu, H.; Wang, T.; Wang, J.; Zhu, X.; Zhu, X.; Zhu, Y.; et al. Comparative genomics of a bovine Mycobacterium tuberculosis isolate and other strains reveals its potential mechanism of bovine adaptation. Front. Microbiol. 2017, 8, 2500. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Brindisi, M.; Vallone, A.; Butini, S.; Campiani, G.; Nannicini, C.; Giuliani, G.; Anzini, M.; Lamponi, S.; Giorgi, G.; et al. Development of potent inhibitors of the Mycobacterium tuberculosis virulence factor ZMP1 and evaluation of their effect on mycobacterial survival inside macrophages. ChemMedChem 2018, 13, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rosales, A.; Provvedi, R.; Torres-Juarez, F.; Enciso-Moreno, J.A.; Hernandez-Pando, R.; Manganelli, R.; Rivas-Santiago, B. LYSX gene is differentially expressed among Mycobacterium tuberculosis strains with different levels of virulence. Tuberculosis 2017, 106, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Romero, F.; Mendoza-Hernandez, G.; Suarez-Guemes, F.; Hernandez-Pando, R.; Castanon-Arreola, M. Secretome profiling of highly virulent Mycobacterium bovis 04-303 strain reveals higher abundance of virulence-associated proteins. Microb. Pathog. 2016, 100, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, E.R.; Frank, A.A.; Orme, I.M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent mycobacterium tuberculosis. Tuber. Lung Dis. 1997, 78, 57–66. [Google Scholar] [CrossRef]
- Hoff, D.R.; Ryan, G.J.; Driver, E.R.; Ssemakulu, C.C.; De Groote, M.A.; Basaraba, R.J.; Lenaerts, A.J. Location of intra- and extracellular M. Tuberculosis populations in lungs of mice and guinea pigs during disease progression and after drug treatment. PLoS ONE 2011, 6, e17550. [Google Scholar] [CrossRef]
- Garcia-Pelayo, M.C.; Bachy, V.S.; Kaveh, D.A.; Hogarth, P.J. BALB/c mice display more enhanced bcg vaccine induced Th1 and Th17 response than C57BL/6 mice but have equivalent protection. Tuberculosis 2015, 95, 48–53. [Google Scholar] [CrossRef]
- Kimmey, J.M.; Campbell, J.A.; Weiss, L.A.; Monte, K.J.; Lenschow, D.J.; Stallings, C.L. The impact of isgylation during Mycobacterium tuberculosis infection in mice. Microbes Infect. 2017, 19, 249–258. [Google Scholar] [CrossRef]
- Coleman, M.T.; Maiello, P.; Tomko, J.; Frye, L.J.; Fillmore, D.; Janssen, C.; Klein, E.; Lin, P.L. Early changes by (18)fluorodeoxyglucose positron emission tomography coregistered with computed tomography predict outcome after Mycobacterium tuberculosis infection in cynomolgus macaques. Infect. Immun. 2014, 82, 2400–2404. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Prince, O.; Cooper, A.; Khader, S.A. Cytokines and chemokines in Mycobacterium tuberculosis infection. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Schwander, S.; Dheda, K. Human lung immunity against mycobacterium tuberculosis: Insights into pathogenesis and protection. Am. J. Respir. Crit. Care Med. 2011, 183, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.; Torres-Garcia, D.; Santos-Mendoza, T.; Rodriguez-Reyna, T.S.; Granados, J.; Yunis, E.J. Cellular and humoral mechanisms involved in the control of tuberculosis. Clin. Dev. Immunol. 2012, 193923. [Google Scholar]
- Greveson, K.; Goodhand, J.; Capocci, S.; Woodward, S.; Murray, C.; Cropley, I.; Hamilton, M.; Lipman, M. Yield and cost effectiveness of mycobacterial infection detection using a simple IGRA-based protocol in uk subjects with inflammatory bowel disease suitable for anti-TNFalpha therapy. J. Crohns Colitis 2013, 7, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J., 3rd; Kaufmann, S.H. Recent advances towards tuberculosis control: Vaccines and biomarkers. J. Intern. Med. 2014, 275, 467–480. [Google Scholar] [CrossRef]
- Jurado, J.O.; Pasquinelli, V.; Alvarez, I.B.; Pena, D.; Rovetta, A.I.; Tateosian, N.L.; Romeo, H.E.; Musella, R.M.; Palmero, D.; Chuluyan, H.E.; et al. IL-17 and ifn-gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J. Leukoc. Biol. 2012, 91, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Fu, R.; Yuan, X.; Shi, C.; Wang, S.; Lu, X.; Ma, Z.; Zhang, X.; Qin, W.; Fan, X. Differential immune responses and protective effects in avirulent mycobacterial strains vaccinated BALB/c mice. Curr. Microbiol. 2015, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Silva, M.V.D.; Barros, C.C.O.; Alexandre, P.B.D.; Timoteo, R.P.; Catarino, J.S.; Sales-Campos, H.; Machado, J.R.; Rodrigues, D.B.R.; Oliveira, C.J.; et al. TNF-alpha blockade impairs in vitro tuberculous granuloma formation and down modulate Th1, Th17 and Treg cytokines. PLoS ONE 2018, 13, e0194430. [Google Scholar]
- Dorhoi, A.; Reece, S.T.; Kaufmann, S.H. For better or for worse: The immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol. Rev. 2011, 240, 235–251. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Dorhoi, A. Inflammation in tuberculosis: Interactions, imbalances and interventions. Curr. Opin. Immunol. 2013, 25, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.F.; Koch, A.; Mizrahi, V. Diversity and disease pathogenesis in mycobacterium tuberculosis. Trends Microbiol. 2015, 23, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kalscheuer, R.; Weinrick, B.; Veeraraghavan, U.; Besra, G.S.; Jacobs, W.R., Jr. Trehalose-recycling abc transporter LpqY-SugA-SugB-SugC is essential for virulence of mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2010, 107, 21761–21766. [Google Scholar] [CrossRef] [PubMed]
- Onwueme, K.C.; Vos, C.J.; Zurita, J.; Ferreras, J.A.; Quadri, L.E. The dimycocerosate ester polyketide virulence factors of mycobacteria. Prog. Lipid Res. 2005, 44, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Hotter, G.S.; Wards, B.J.; Mouat, P.; Besra, G.S.; Gomes, J.; Singh, M.; Bassett, S.; Kawakami, P.; Wheeler, P.R.; de Lisle, G.W.; et al. Transposon mutagenesis of MB0100 at the ppe1-nrp locus in Mycobacterium bovis disrupts phthiocerol dimycocerosate (PDIM) and glycosylphenol-PDIM biosynthesis, producing an avirulent strain with vaccine properties at least equal to those of M. Bovis BCG. J. Bacteriol. 2005, 187, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, O.; Miyata, T.; Goodridge, A.; Kendall, L.V.; Riley, L.W. Mce2 operon mutant strain of Mycobacterium tuberculosis is attenuated in C57BL/6 mice. Tuberculosis 2010, 90, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kirksey, M.A.; Tischler, A.D.; Simeone, R.; Hisert, K.B.; Uplekar, S.; Guilhot, C.; McKinney, J.D. Spontaneous phthiocerol dimycocerosate-deficient variants of Mycobacterium tuberculosis are susceptible to gamma interferon-mediated immunity. Infect. Immun. 2011, 79, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Bottai, D.; Majlessi, L.; Simeone, R.; Frigui, W.; Laurent, C.; Lenormand, P.; Chen, J.; Rosenkrands, I.; Huerre, M.; Leclerc, C.; et al. ESAT-6 secretion-independent impact of ESX-1 genes espf and espg1 on virulence of mycobacterium tuberculosis. J. Infect. Dis. 2011, 203, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Xu, D.; Wang, J.; Liu, C.; Zhou, Y.; Cui, Y.; Liu, H.; Wan, K.; Zhou, X. Isolation and identification of multiple drug resistant nontuberculous mycobacteria from organs of cattle produced typical granuloma lesions. Microb. Pathog. 2017, 107, 313–316. [Google Scholar] [CrossRef]
- Chen, Y.; Chao, Y.; Deng, Q.; Liu, T.; Xiang, J.; Chen, J.; Zhou, J.; Zhan, Z.; Kuang, Y.; Cai, H.; et al. Potential challenges to the stop tb plan for humans in china; cattle maintain M. Bovis and M. Tuberculosis. Tuberculosis 2009, 89, 95–100. [Google Scholar] [CrossRef]


| SNP | InDel | ||
|---|---|---|---|
| Intergenic region | 86 | Conservative inframe deletion | 13 |
| Missense variant | 409 | Disruptive inframe insertion | 26 |
| Stop gained | 7 | Frameshift variant | 60 |
| Stop lost & splice region variant | 2 | Frameshift variant & stop gained | 4 |
| Synonymous variant | 246 | Intergenic region | 31 |
| Pilon deletion | 11 | ||
| Gene Mb# | VFclass | Virulence factors | Strain C68004 | Strain N |
|---|---|---|---|---|
| Mb0607 | Mammalian cell entry (mce) operons | Mce2 | - | mce2D |
| Mb1214 | Cell surface components | Glycopeptidolipid (GPL) locus | - | papA3 |
| Mb3856 | Cell surface components | Trehalose-recycling ATP-binding cassette (ABC) transporter | fad23 | - |
| Mb3906 | Secretion system | ESAT-6 secretion system (ESX)-1 (T7SS) | espI | - |
| Mb3916c-Mb3917c | Secretion system | ESX-2 (T7SS) | - | mycP2 |
| Secretion system | ESX-2 (T7SS) | - | eccD2 | |
| Mb3924C | Secretion system | ESX-2 (T7SS) | eccC2 | - |
| Mb0208C | Metal uptake | Heme uptake | - | mmpL11 |
| Mb0250C | Cell surface components | GPL locus | - | fadE5 |
| Mb0661C | Cell surface components | Methyltransferase | mmaA4 | - |
| Mb0994 | Metal exporters | Copper exporter | - | ctpV |
| Mb1193 | Anaerobic respiration | Nitrate reductase | - | narG |
| Mb1267 | Cell surface components | Trehalose-recycling ABC transporter | - | lpqY |
| Mb1553C | Cell surface components | GPL locus | - | gtf1 |
| Mb1766C | Anaerobic respiration | Nitrate/nitrite transporter | narK2 | - |
| Mb2139C | Proteases | Proteasome-associated proteins | mpa | - |
| Mb2404C | Metal uptake | Mycobactin | - | mbtB |
| Mb2439C | Secreted proteins | Enhanced intracellular survival protein | - | eis |
| Mb2958 | Cell surface components | Phthiocerol dimycocerosate (PDIM) and Phenolic glycolipid (PGL) biosynthesis and transport | - | ppsC |
| Mb2959 | Cell surface components | PDIM and PGL biosynthesis and transport | - | ppsD |
| Mb2960 | Cell surface components | PDIM and PGL biosynthesis and transport | - | ppsE |
| Mb2966 | Cell surface components | PDIM and PGL biosynthesis and transport | - | fadD28 |
| Mb2982C | Cell surface components | PDIM and PGL biosynthesis and transport | - | - |
| Mb3298 | Metal exporters | P(1)-type Mn2+ transporting ATPase | - | ctpC |
| Mb3631C | Lipid and fatty acid metabolism | Pantothenate synthesis | panD | - |
| Mb3851 | Cell surface components | Trehalose-recycling ABC transporter | sap | - |
| Mb3896 | Regulation | RegX3 | espG1 | - |
| Mb3912C | Secretion system | ESX-1 (T7SS) | - | eccE1 |
| Mb0606 | mce operons | Mce2 | - | mce2C |
| Mb0609 | mce operons | Mce2 | - | mce2E |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Hussain, T.; Sabir, N.; Ni, J.; Li, M.; Zhao, D.; Zhou, X. Comparative Study of the Molecular Basis of Pathogenicity of M. bovis Strains in a Mouse Model. Int. J. Mol. Sci. 2019, 20, 5. https://doi.org/10.3390/ijms20010005
Cheng G, Hussain T, Sabir N, Ni J, Li M, Zhao D, Zhou X. Comparative Study of the Molecular Basis of Pathogenicity of M. bovis Strains in a Mouse Model. International Journal of Molecular Sciences. 2019; 20(1):5. https://doi.org/10.3390/ijms20010005
Chicago/Turabian StyleCheng, Guangyu, Tariq Hussain, Naveed Sabir, Jiamin Ni, Miaoxuan Li, Deming Zhao, and Xiangmei Zhou. 2019. "Comparative Study of the Molecular Basis of Pathogenicity of M. bovis Strains in a Mouse Model" International Journal of Molecular Sciences 20, no. 1: 5. https://doi.org/10.3390/ijms20010005




