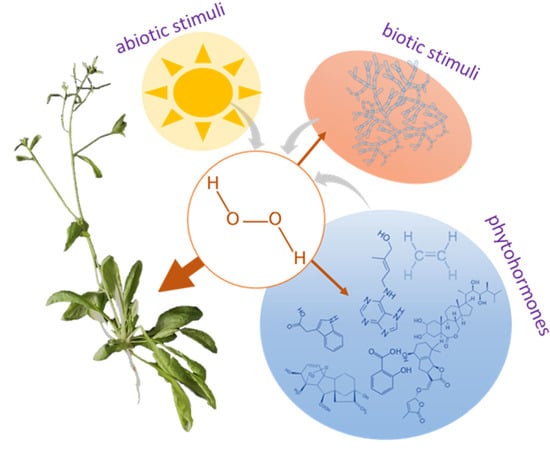
Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks
Abstract
:1. Introduction
2. Metabolism
2.1. Electron. Transport Chains and Superoxide Dismutase
2.2. NADPH Oxidase
2.3. Polyamine Oxidase
2.4. Peroxisomal Production of H2O2
2.5. The H2O2 Scavenging System
2.6. Catalases
2.7. Ascorbate and Thiol-Specific Peroxidases
2.8. Peroxidases (Class III)
3. Transport
Peroxiporins
4. Signalling
4.1. Oxidation of Cysteine Residues
4.2. Oxidation of Methionine Residues
4.3. Other Protein PTMs
4.4. Transcription Factors
4.4.1. HsfA
4.4.2. NAC Domain-Containing Protein
4.4.3. Mediators of RNA Polymerase
4.4.4. WRKY and ZAT (Zinc finger of Arabidopsis thaliana) Transcription Factors
4.5. Calcium Ions
5. H2O2 in Growth and Development
5.1. The Crosstalk between H2O2 and Phytohormones
5.2. Light Signalling
5.3. Dry Seed
5.4. Germination
5.5. Root Development
5.6. Shoot Development
5.7. Stomatal Movement
5.8. Pollination
5.9. Fruit Ripening
5.10. Senescence and Cell Death
5.11. Stress
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Möller, D. Atmospheric hydrogen peroxide: Evidence for aqueous-phase formation from a historic perspective and a one-year measurement campaign. Atmos. Environ. 2009, 43, 5923–5936. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrzaczek, M.; Brosché, M.; Kangasjärvi, J. ROS signalling loops—Production, perception, regulation. Curr. Opin. Plant Biol. 2013, 16, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Bloom, A.J.; Queval, G.; Noctor, G. Photorespiratory Metabolism: Genes, Mutants, Energetics and Redox Signalling. Annu. Rev. Plant Biol. 2009, 60, 455–484. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Mashiguchi, K.; Chen, Q.; Kasahara, H.; Kamiya, Y.; Ojha, S.; DuBois, J.; Ballou, D.; Zhao, Y. The biochemical mechanism of auxin biosynthesis by an arabidopsis YUCCA flavin-containing monooxygenase. J. Biol. Chem. 2013, 288, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Siddens, L.K.; Krueger, S.K.; Henderson, M.C.; Williams, D.E. Mammalian flavin-containing monooxygenase (FMO) as a source of hydrogen peroxide. Biochem. Pharmacol. 2014, 89, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.-J.; Turkan, I.; Krieger-Liszkay, A. Redox- and Reactive Oxygen Species-Dependent Signaling into and out of the Photosynthesizing Chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Khorobrykh, S.A.; Karonen, M.; Tyystjärvi, E. Experimental evidence suggesting that H2O2 is produced within the thylakoid membrane in a reaction between plastoquinol and singlet oxygen. FEBS Lett. 2015, 589, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The Roles of Mitochondrial Reactive Oxygen Species in Cellular Signaling and Stress Response in Plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M. Plant Mitochondria and Oxidative Stress: Electron Transport, NADPH Turnover and Metabolism of Reactive Oxygen Species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—Mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]
- Tanz, S.K.; Castleden, I.; Hooper, C.M.; Vacher, M.; Small, I.; Millar, H.A. SUBA3: A database for integrating experimentation and prediction to define the SUBcellular location of proteins in Arabidopsis. Nucleic Acids Res. 2012, 41, D1185–D1191. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, V.; Hanlon, M.R.; Contrino, S.; Ferlanti, E.S.; Karamycheva, S.; Kim, M.; Rosen, B.D.; Cheng, C.-Y.; Moreira, W.; Mock, S.A.; et al. Araport: The Arabidopsis Information Portal. Nucleic Acids Res. 2015, 43, D1003–D1009. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.; Gilroy, S. ROS in plant development. Physiol. Plant. 2010, 138, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.-W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.-G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Yan, M.; Zhang, Q. Functional regulation of plant NADPH oxidase and its role in signaling. Plant Signal. Behav. 2017, 12, e1356970. [Google Scholar] [CrossRef] [PubMed]
- Yoda, H.; Yamaguchi, Y.; Sano, H. Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol. 2003, 132, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-Containing Amine Oxidases and FAD-Dependent Polyamine Oxidases Are Key Players in Plant Tissue Differentiation and Organ Development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Fincato, P.; Moschou, P.N.; Spedaletti, V.; Tavazza, R.; Angelini, R.; Federico, R.; Roubelakis-Angelakis, K.A.; Tavladoraki, P. Functional diversity inside the Arabidopsis polyamine oxidase gene family. J. Exp. Bot. 2011, 62, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sengupta, A.; Chakraborty, M.; Gupta, B. Hydrogen Peroxide and Polyamines Act as Double Edged Swords in Plant Abiotic Stress Responses. Front. Plant Sci. 2016, 7, 1343. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Kuklová, A.; Hoehenwarter, W.; Fragner, L.; Novák, O.; Rotková, G.; Jedelský, P.L.; Žáková, K.; Šmehilová, M.; Strnad, M.; et al. Proteome and metabolome profiling of cytokinin action in Arabidopsis identifying both distinct and similar responses to cytokinin down- and up-regulation. J. Exp. Bot. 2013, 64, 4193–4206. [Google Scholar] [CrossRef] [PubMed]
- Hesberg, C.; Hänsch, R.; Mendel, R.R.; Bittner, F. Tandem Orientation of Duplicated Xanthine Dehydrogenase Genes from Arabidopsis thaliana: Differential gene expression and enzyme activities. J. Biol. Chem. 2004, 279, 13547–13554. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Baker, A.; Bartel, B.; Linka, N.; Mullen, R.T.; Reumann, S.; Zolman, B.K. Plant Peroxisomes: Biogenesis and Function. Plant Cell 2012, 24, 2279–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Maurino, V.G.; Peterhansel, C. Photorespiration: Current status and approaches for metabolic engineering. Curr. Opin. Plant Biol. 2010, 13, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Drago, I.; Behera, S.; Zottini, M.; Pizzo, P.; Schroeder, J.I.; Pozzan, T.; Schiavo, F.L. H2O2 in plant peroxisomes: An in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J. 2010, 62, 760–772. [Google Scholar] [CrossRef] [PubMed]
- del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide—A central hub for information flow in plant cells. AoB Plants 2012, 2012, pls014. [Google Scholar] [CrossRef] [PubMed]
- Ksas, B.; Légeret, B.; Ferretti, U.; Chevalier, A.; Pospíšil, P.; Alric, J.; Havaux, M. The plastoquinone pool outside the thylakoid membrane serves in plant photoprotection as a reservoir of singlet oxygen scavengers. Plant Cell Environ. 2018. [Google Scholar] [CrossRef] [PubMed]
- Khorobrykh, S.; Tyystjärvi, E. Plastoquinol generates and scavenges reactive oxygen species in organic solvent: Potential relevance for thylakoids. Biochim. Biophys. Acta-Bioenerg. 2018, 1859, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.; Simonian, R.; Skulachev, V.; Starkov, A. Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Lett. 1997, 415, 87–90. [Google Scholar] [CrossRef]
- Wiciarz, M.; Gubernator, B.; Kruk, J.; Niewiadomska, E. Enhanced chloroplastic generation of H2O2 in stress-resistant Thellungiella salsuginea in comparison to Arabidopsis thaliana. Physiol. Plant. 2015, 153, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Mishra, P.; Das, P.; Sabat, S.C. Expression and purification of soluble bio-active rice plant catalase-A from recombinant Escherichia coli. J. Biotechnol. 2012, 157, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Kitamura, M.; Koja, N. Triple mutation of Cys26, Trp35 and Cys126 in stromal ascorbate peroxidase confers H2O2 tolerance comparable to that of the cytosolic isoform. Biochem. Biophys. Res. Commun. 2008, 372, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Doubnerová, V.; Müller, K.; Ryšlavá, H. Characterization of phosphoenolpyruvate carboxylase from mature maize seeds: Properties of phosphorylated and dephosphorylated forms. Biochimie 2010, 92, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2005, 17, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, D.E.; Koc, A.; Agisheva, N.; Jacobsen, M.; Kaya, A.; Malinouski, M.; Rutherford, J.C.; Siu, K.-L.; Jin, D.-Y.; Winge, D.R.; et al. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc. Natl. Acad. Sci. USA 2011, 108, 2729–2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebthal, M.; Maynard, D.; Dietz, K.-J. Peroxiredoxins and Redox Signaling in Plants. Antioxid. Redox Signal. 2018, 28, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Podgórska, A.; Burian, M.; Szal, B. Extra-Cellular But Extra-Ordinarily Important for Cells: Apoplastic Reactive Oxygen Species Metabolism. Front. Plant Sci. 2017, 8, 1353. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Daudi, A.; Finch, P.; Butt, V.S.; Whitelegge, J.P.; Souda, P.; Ausubel, F.M.; Bolwell, G.P. A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 2012, 158, 2013–2027. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, S.V.; Kukavica, B.; Vidović, M.; Morina, F.; Menckhoff, L. Class III Peroxidases: Functions, Localization and Redox Regulation of Isoenzymes. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing: Cham, Switzerland, 2018; pp. 269–300. [Google Scholar]
- Tewari, R.K.; Singh, P.K.; Watanabe, M. The spatial patterns of oxidative stress indicators co-locate with early signs of natural senescence in maize leaves. Acta Physiol. Plant. 2013, 35, 949–957. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological Production, Detection and Fate of Hydrogen Peroxide. Antioxid. Redox Signal. 2017, 29, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Stöcker, S.; Van Laer, K.; Mijuskovic, A.; Dick, T.P. The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs. Antioxid. Redox Signal. 2018, 28, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Henzler, T.; Steudle, E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: Model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J. Exp. Bot. 2000, 51, 2053–2066. [Google Scholar] [CrossRef] [PubMed]
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and evolution of membrane intrinsic proteins. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 1468–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooijmaijers, C.; Rhee, J.Y.; Kwak, K.J.; Chung, G.C.; Horie, T.; Katsuhara, M.; Kang, H. Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J. Plant Res. 2012, 125, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Heinen, R.B.; Berny, M.C.; Chaumont, F. Maize plasma membrane aquaporin ZmPIP2;5 but not ZmPIP1;2, facilitates transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, M.; Sasano, S.; Horie, T.; Matsumoto, T.; Rhee, J.; Shibasaka, M. Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotechnol. 2014, 31, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide across Membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, X.; Li, P.; Wang, H.; Ji, H.; Xie, J.; Qiu, Q.; Shen, D.; Dong, H. Plant Aquaporin AtPIP1;4 Links Apoplastic H2O2 Induction to Disease Immunity Pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Yoshikawa, N.; Ishikawa, T.; Sawa, Y.; Shibata, H. Substitution of a single amino acid residue in the aromatic/arginine selectivity filter alters the transport profiles of tonoplast aquaporin homologs. Biochim. Biophys. Acta 2012, 1818, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienert, G.P.; Bienert, M.D.; Jahn, T.P.; Boutry, M.; Chaumont, F. Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J. 2011, 66, 306–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.X.; Steudle, E. Gating of aquaporins by light and reactive oxygen species in leaf parenchyma cells of the midrib of Zea mays. J. Exp. Bot. 2009, 60, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Verdoucq, L.; Rodrigues, O.; Martinière, A.; Luu, D.T.; Maurel, C. Plant aquaporins on the move: Reversible phosphorylation, lateral motion and cycling. Curr. Opin. Plant Biol. 2014, 22, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Skalák, J.; Cerna, H.; Brzobohatý, B. Advances in purification and separation of posttranslationally modified proteins. J. Proteomics 2013, 92, 2–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-W.; Helmann, J.D. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 2006, 440, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Andronis, E.A.; Moschou, P.N.; Toumi, I.; Roubelakis-Angelakis, K.A. Peroxisomal polyamine oxidase and NADPH-oxidase cross-talk for ROS homeostasis which affects respiration rate in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Sabater, B.; Martín, M. Hypothesis: Increase of the ratio singlet oxygen plus superoxide radical to hydrogen peroxide changes stress defense response to programmed leaf death. Front. Plant Sci. 2013, 4, 479. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Chibani, K.; Jacquot, J.-P.; Rouhier, N. Cysteine–based redox regulation and signaling in plants. Front. Plant Sci. 2013, 4, 105. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.-C.; Chen, J.; Miao, C.; Song, C.-P. An Arabidopsis Glutathione Peroxidase Functions as Both a Redox Transducer and a Scavenger in Abscisic Acid and Drought Stress Responses. Plant Cell Online 2006, 18, 2749–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuramalingam, M.; Matros, A.; Scheibe, R.; Mock, H.-P.; Dietz, K.-J. The hydrogen peroxide-sensitive proteome of the chloroplast in vitro and in vivo. Front. Plant Sci. 2013, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-W.; Lee, S.-H.; Chieh, P.-S.; Lin, C.-S.; Wang, Y.-C.; Chan, M.-T. Arabidopsis Root-Abundant Cytosolic Methionine Sulfoxide Reductase B Genes MsrB7 and MsrB8 are Involved in Tolerance to Oxidative Stress. Plant Cell Physiol. 2012, 53, 1707–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, S.; Ghesquière, B.; De Bock, P.-J.; Demol, H.; Wahni, K.; Willems, P.; Messens, J.; Van Breusegem, F.; Gevaert, K. Protein Methionine Sulfoxide Dynamics in Arabidopsis thaliana under Oxidative Stress. Mol. Cell. Proteomics 2015, 14, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Hardin, S.C.; Larue, C.T.; Oh, M.-H.; Jain, V.; Huber, S.C. Coupling oxidative signals to protein phosphorylation via methionine oxidation in Arabidopsis. Biochem. J. 2009, 422, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, D.; Alvim Kamei, C.L.; Cools, T.; Vanderauwera, S.; Takahashi, N.; Okushima, Y.; Eekhout, T.; Yoshiyama, K.O.; Larkin, J.; Van den Daele, H.; et al. The Arabidopsis SIAMESE-RELATED cyclin-dependent kinase inhibitors SMR5 and SMR7 regulate the DNA damage checkpoint in response to reactive oxygen species. Plant Cell 2014, 26, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zentgraf, U. A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J. 2010, 63, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Černý, M.; Novák, J.; Habánová, H.; Cerna, H.; Brzobohatý, B. Role of the proteome in phytohormonal signaling. Biochim. Biophys. Acta-Proteins Proteom. 2016, 1864, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Schöffl, F.; Rieping, M.; Baumann, G.; Bevan, M.; Angermüller, S. The function of plant heat shock promoter elements in the regulated expression of chimaeric genes in transgenic tobacco. Mol. Gen. Genet. 1989, 217, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Panchuk, I.I.; Volkov, R.A.; Schöffl, F. Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 2002, 129, 838–853. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Mittler, R. Could Heat Shock Transcription Factors Function as Hydrogen Peroxide Sensors in Plants? Ann. Bot. 2006, 98, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishizawa, A.; Yabuta, Y.; Yoshida, E.; Maruta, T.; Yoshimura, K.; Shigeoka, S. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006, 48, 535–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balazadeh, S.; Wu, A.; Mueller-Roeber, B. Salt-triggered expression of the ANAC092-dependent senescence regulon in Arabidopsis thaliana. Plant Signal. Behav. 2010, 5, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Allu, A.D.; Garapati, P.; Siddiqui, H.; Dortay, H.; Zanor, M.-I.; Asensi-Fabado, M.A.; Munné-Bosch, S.; Antonio, C.; Tohge, T.; et al. JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 2012, 24, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Balazadeh, S.; Kwasniewski, M.; Caldana, C.; Mehrnia, M.; Zanor, M.I.; Xue, G.-P.; Mueller-Roeber, B. ORS1, an H2O2-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana. Mol. Plant 2011, 4, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Ivanova, A.; Duncan, O.; Law, S.R.; Van Aken, O.; De Clercq, I.; Wang, Y.; Carrie, C.; Xu, L.; Kmiec, B.; et al. A Membrane-Bound NAC Transcription Factor, ANAC017, Mediates Mitochondrial Retrograde Signaling in Arabidopsis. Plant Cell 2013, 25, 3450–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, I.; Vermeirssen, V.; Van Aken, O.; Vandepoele, K.; Murcha, M.W.; Law, S.R.; Inzé, A.; Ng, S.; Ivanova, A.; Rombaut, D.; et al. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 2013, 25, 3472–3490. [Google Scholar] [CrossRef] [PubMed]
- Shaikhali, J.; Davoine, C.; Brännström, K.; Rouhier, N.; Bygdell, J.; Björklund, S.; Wingsle, G. Biochemical and redox characterization of the mediator complex and its associated transcription factor GeBPL, a GLABROUS1 enhancer binding protein. Biochem. J. 2015, 468, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Shaikhali, J.; Davoine, C.; Björklund, S.; Wingsle, G. Redox regulation of the MED28 and MED32 mediator subunits is important for development and senescence. Protoplasma 2016, 253, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY Transcription Factors: Molecular Regulation and Stress Responses in Plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.J.; Yan, J.Y.; Xu, X.Y.; Yu, D.Q.; Li, G.X.; Zhang, S.Q.; Zheng, S.J. Transcription factor WRKY46 regulates osmotic stress responses and stomatal movement independently in Arabidopsis. Plant J. 2014, 79, 13–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciftci-Yilmaz, S.; Morsy, M.R.; Song, L.; Coutu, A.; Krizek, B.A.; Lewis, M.W.; Warren, D.; Cushman, J.; Connolly, E.L.; Mittler, R. The EAR-motif of the Cys2/His2-type zinc finger protein Zat7 plays a key role in the defense response of Arabidopsis to salinity stress. J. Biol. Chem. 2007, 282, 9260–9268. [Google Scholar] [CrossRef] [PubMed]
- Le, C.T.T.; Brumbarova, T.; Ivanov, R.; Stoof, C.; Weber, E.; Mohrbacher, J.; Fink-Straube, C.; Bauer, P. ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) Interacts with FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) Linking Iron Deficiency and Oxidative Stress Responses. Plant Physiol. 2016, 170, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Pavet, V.; Olmos, E.; Kiddle, G.; Mowla, S.; Kumar, S.; Antoniw, J.; Alvarez, M.E.; Foyer, C.H. Ascorbic Acid Deficiency Activates Cell Death and Disease Resistance Responses in Arabidopsis. Plant Physiol. 2005, 139, 1291–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scrase-Field, S.A.M.G.; Knight, M.R. Calcium: Just a chemical switch? Curr. Opin. Plant Biol. 2003, 6, 500–506. [Google Scholar] [CrossRef]
- Peiter, E. The Ever-Closer Union of Signals: Propagating Waves of Calcium and ROS Are Inextricably Linked. Plant Physiol. 2016, 172, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Z.-M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Poovaiah, B.W. Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandadi, K.K.; Misra, A.; Ren, S.; McKnight, T.D. BT2, a BTB protein, mediates multiple responses to nutrients, stresses and hormones in Arabidopsis. Plant Physiol. 2009, 150, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Vierstra, R.D. The Cullin-RING Ubiquitin-Protein Ligases. Annu. Rev. Plant Biol. 2011, 62, 299–334. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Brown, I.; Adams, S.; Knight, M.; Ainslie, A.; Mansfield, J. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000, 23, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Inzé, D.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, R.; Hancock, J.T.; Bright, J.; Harrison, J.; Weir, I.; Hooley, R.; Neill, S.J. A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol. 2005, 137, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of Indole-3-Butyric Acid Homeostasis by the UDP-Glucosyltransferase UGT74E2 Modulates Arabidopsis Architecture and Water Stress Tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Wu, J.; Yuan, D.; Zhang, D.; Huang, Z.; Xiao, L.; Yang, C. Perturbation of auxin homeostasis caused by mitochondrial FtSH4 gene-mediated peroxidase accumulation regulates arabidopsis architecture. Mol. Plant. 2014, 7, 856–873. [Google Scholar] [CrossRef] [PubMed]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, S.; Han, C.; Nanjo, Y.; Altaf-Un-Nahar, M.; Wang, K.; He, D.; Yang, P. Label-free quantitative proteomic analysis of abscisic acid effect in early-stage soybean under flooding. J. Proteome Res. 2013, 12, 4769–4784. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, A.; Baerenfaller, K.; Loscos, J.; Chételat, A.; Baginsky, S.; Farmer, E.E. Jasmonate controls polypeptide patterning in undamaged tissue in wounded Arabidopsis leaves. Plant Physiol. 2011, 156, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Jedelský, P.L.; Novák, J.; Schlosser, A.; Brzobohatý, B. Cytokinin modulates proteomic, transcriptomic and growth responses to temperature shocks in Arabidopsis. Plant Cell Environ. 2014, 37, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Žd’árská, M.; Zatloukalová, P.; Benítez, M.; Šedo, O.; Potěšil, D.; Novák, O.; Svačinová, J.; Pešek, B.; Malbeck, J.; Vašíčková, J.; et al. Proteome analysis in Arabidopsis reveals shoot- and root-specific targets of cytokinin action and differential regulation of hormonal homeostasis. Plant Physiol. 2013, 161, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Dai, S.; Zhu, N.; Booy, A.; Simons, B.; Yi, S.; Chen, S. Methyl jasmonate responsive proteins in Brassica napus guard cells revealed by iTRAQ-based quantitative proteomics. J. Proteome Res. 2012, 11, 3728–3742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, N.; Song, W.; Harmon, A.C.; Assmann, S.M.; Chen, S. Thiol-based redox proteins in abscisic acid and methyl jasmonate signaling in Brassica napus guard cells. Plant J. 2014, 78, 491–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.; Blackburn, K.; Lin, Y.; Goshe, M.B.; Williamson, J.D. Absolute protein quantification by LC/MS(E) for global analysis of salicylic acid-induced plant protein secretion responses. J. Proteome Res. 2009, 8, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Takahashi, D.; Kawamura, Y.; Uemura, M. Comparison of plasma membrane proteomic changes of Arabidopsis suspension-cultured cells (T87 Line) after cold and ABA treatment in association with freezing tolerance development. Plant Cell Physiol. 2012, 53, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Lochmanová, G.; Zdráhal, Z.; Konečná, H.; Koukalová, Š.; Malbeck, J.; Souček, P.; Válková, M.; Kiran, N.S.; Brzobohatý, B. Cytokinin-induced photomorphogenesis in dark-grown Arabidopsis: A. proteomic analysis. J. Exp. Bot. 2008, 59, 3705–3719. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Bertini, L.; Timperio, A.M.; Zolla, L.; Caporale, C.; Caruso, C. Crosstalk between salicylic acid and jasmonate in Arabidopsis investigated by an integrated proteomic and transcriptomic approach. Mol. Biosyst. 2013, 9, 1169–1187. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, M.; Schroeder, J.I. Quantitative transcriptomic analysis of abscisic acid-induced and reactive oxygen species-dependent expression changes and proteomic profiling in Arabidopsis suspension cells. Plant J. 2011, 67, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Dai, S.Y.; Yuan, J.S. Integration of shot-gun proteomics and bioinformatics analysis to explore plant hormone responses. BMC Bioinform. 2012, 13, S8. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Xue, H. A proteomics study of auxin effects in Arabidopsis thaliana. Acta Biochim. Biophys. Sin. (Shanghai) 2012, 44, 783–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueckershoff, K.; Mueller, S.; Mueller, M.J.; Reinders, J. Impact of cyclopentenone-oxylipins on the proteome of Arabidopsis thaliana. Biochim. Biophys. Acta 2008, 1784, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Dyčka, F.; Bobál′ová, J.; Brzobohatý, B. Early cytokinin response proteins and phosphoproteins of Arabidopsis thaliana identified by proteome and phosphoproteome profiling. J. Exp. Bot. 2011, 62, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Belghazi, M.; Huguet, R.; Robin, C.; Moreau, A.; Job, C.; Job, D. Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol. 2006, 141, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Czarnecki, O.; Chourey, K.; Yang, J.; Tuskan, G.A.; Hurst, G.B.; Pan, C.; Chen, J.-G. Strigolactone-regulated proteins revealed by iTRAQ-based quantitative proteomics in Arabidopsis. J. Proteome Res. 2014, 13, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, T.; Yasuda, D.; Mori, T.; Yoshimitsu, Y.; Nakamura, Y.; Yoshida, S.; Asami, T.; Okamoto, S.; Matsuo, T. Characterization of brassinosteroid-regulated proteins in a nuclear-enriched fraction of Arabidopsis suspension-cultured cells. Plant Physiol. Biochem. 2011, 49, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xue, L.; Batelli, G.; Lee, S.; Hou, Y.-J.; Van Oosten, M.J.; Zhang, H.; Tao, W.A.; Zhu, J.-K. Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc. Natl. Acad. Sci. USA 2013, 110, 11205–11210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jourdan, N.; Martino, C.F.; El-Esawi, M.; Witczak, J.; Bouchet, P.-E.; d’Harlingue, A.; Ahmad, M. Blue-light dependent ROS formation by Arabidopsis cryptochrome-2 may contribute toward its signaling role. Plant Signal. Behav. 2015, 10, e1042647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Esawi, M.; Arthaut, L.-D.; Jourdan, N.; d’Harlingue, A.; Link, J.; Martino, C.F.; Ahmad, M. Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 2017, 7, 13875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consentino, L.; Lambert, S.; Martino, C.; Jourdan, N.; Bouchet, P.-E.; Witczak, J.; Castello, P.; El-Esawi, M.; Corbineau, F.; d’Harlingue, A.; et al. Blue-light dependent reactive oxygen species formation by Arabidopsis cryptochrome may define a novel evolutionarily conserved signaling mechanism. New Phytol. 2015, 206, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-H.; Kim, J.-H.; Kim, S.-G.; Sim, H.-J.; Lee, G.; Halitschke, R.; Baldwin, I.T.; Kim, J.-I.; Park, C.-M. Shoot phytochrome B modulates reactive oxygen species homeostasis in roots via abscisic acid signaling in Arabidopsis. Plant J. 2018, 94, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. C. R. Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; He, D.; Gupta, R.; Yang, P. Physiological and proteomic analyses on artificially aged Brassica napus seed. Front. Plant Sci. 2015, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- KOCSY, G. Die or survive? Redox changes as seed viability markers. Plant Cell Environ. 2015, 38, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.-P.; Cueff, G.; Hegedus, D.D.; Rajjou, L.; Bentsink, L. A role for seed storage proteins in Arabidopsis seed longevity. J. Exp. Bot. 2015, 66, 6399–6413. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Garcia, Q. Reactive oxygen species and seed germination. Biologia 2013, 68, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, B.; Xu, Z.; Shi, Z.; Chen, S.; Huang, X.; Chen, J.; Wang, X. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. J. Exp. Bot. 2014, 65, 3189–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oracz, K.; Karpiński, S. Phytohormones Signaling Pathways and ROS Involvement in Seed Germination. Front. Plant Sci. 2016, 7, 864. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Watabe, G.; Yuasa, T.; Iwaya-Inoue, M.; Ishibashi, Y. Role of reactive oxygen species produced by NADPH oxidase in gibberellin biosynthesis during barley seed germination. Plant Signal. Behav. 2016, 11, e1180492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, Y.; Aoki, N.; Kasa, S.; Sakamoto, M.; Kai, K.; Tomokiyo, R.; Watabe, G.; Yuasa, T.; Iwaya-Inoue, M. The Interrelationship between Abscisic Acid and Reactive Oxygen Species Plays a Key Role in Barley Seed Dormancy and Germination. Front. Plant Sci. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Lariguet, P.; Ranocha, P.; De Meyer, M.; Barbier, O.; Penel, C.; Dunand, C. Identification of a hydrogen peroxide signalling pathway in the control of light-dependent germination in Arabidopsis. Planta 2013, 238, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different Modes of Hydrogen Peroxide Action During Seed Germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal. Behav. 2012, 7, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf-Bouteau, H.; Bailly, C. Targeted mRNA Oxidation Regulates Sunflower Seed Dormancy Alleviation during Dry After-Ripening. Plant Cell 2011, 23, 2196–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maarouf-Bouteau, H.; Meimoun, P.; Job, C.; Job, D.; Bailly, C. Role of protein and mRNA oxidation in seed dormancy and germination. Front. Plant Sci. 2013, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A Role for Reactive Oxygen Species Produced by NADPH Oxidases in the Embryo and Aleurone Cells in Barley Seed Germination. PLoS ONE 2015, 10, e0143173. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Ishibashi, Y.; Kai, K.; Tomokiyo, R.; Yuasa, T.; Iwaya-Inoue, M. Programmed cell death in barley aleurone cells is not directly stimulated by reactive oxygen species produced in response to gibberellin. J. Plant Physiol. 2014, 171, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Tawaratsumida, T.; Kondo, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Zheng, S.-H.; Yuasa, T.; Iwaya-Inoue, M. Reactive Oxygen Species Are Involved in Gibberellin/Abscisic Acid Signaling in Barley Aleurone Cells. Plant Physiol. 2012, 158, 1705–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farouk, S.; Abdul Qados, A.M.S. Enhancing seed quality and productivity as well as physio-anatomical responses of pea plants by folic acid and/or hydrogen peroxide application. Sci. Hortic. (Amsterdam) 2018, 240, 29–37. [Google Scholar] [CrossRef]
- Bouallègue, A.; Souissi, F.; Nouairi, I.; Souibgui, M.; Abbes, Z.; Mhadhbi, H. Salicylic acid and hydrogen peroxide pretreatments alleviate salt stress in faba bean (Vicia faba) seeds during germination. Seed Sci. Technol. 2017, 45, 675–690. [Google Scholar] [CrossRef]
- Li, B.; Cai, Q.; Ma, S.; Li, S.; Zhang, X.; Yu, Y. Regulation of NPA and ACC on H2O2-Induced Pea Primary Horizontal Bending Root. J. Plant Growth Regul. 2018, 37, 246–254. [Google Scholar] [CrossRef]
- Carol, R.J.; Takeda, S.; Linstead, P.; Durrant, M.C.; Kakesova, H.; Derbyshire, P.; Drea, S.; Zarsky, V.; Dolan, L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 2005, 438, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, Q.; Guo, J.; Wang, P.; Song, P.; Jia, Q.; Zhang, X.; Kudla, J.; Zhang, W.; Zhang, Q. Peroxisomal CuAOζ and its product H2O2 regulate the distribution of auxin and IBA-dependent lateral root development in Arabidopsis. J. Exp. Bot. 2017, 68, 4851–4867. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liu, L.; Liu, H.; Ferguson, B.J.; Zou, Y.; Zhao, Y.; Wang, T.; Wang, Y.; Li, X. H2O2 regulates root system architecture by modulating the polar transport and redistribution of auxin. J. Plant Biol. 2016, 59, 260–270. [Google Scholar] [CrossRef]
- Takáč, T.; Obert, B.; Rolčík, J.; Šamaj, J. Improvement of adventitious root formation in flax using hydrogen peroxide. New Biotechnol. 2016, 33, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gu, Q.; Yu, X.; Huang, L.; Xu, S.; Wang, R.; Shen, W.; Shen, W. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann. Bot. 2018, 121, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Yang, Y.; Fu, G.; Tao, L. Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ivanchenko, M.G.; den Os, D.; Monshausen, G.B.; Dubrovsky, J.G.; Bednárová, A.; Krishnan, N. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum lycopersicum) root tips while inhibiting root growth. Ann. Bot. 2013, 112, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hou, H.; Yang, T.; Lian, Y.; Sun, Y.; Bian, Z.; Wang, C. Exogenous hydrogen peroxide inhibits primary root gravitropism by regulating auxin distribution during Arabidopsis seed germination. Plant Physiol. Biochem. PPB 2018, 128, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Sun, L.; Song, Y.; Wang, L.; Liu, L.; Zhang, L.; Liu, B.; Li, N.; Miao, C.; Hao, F. AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. J. Exp. Bot. 2013, 64, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yan, J.; Yu, X.; Liang, Y.; Fang, L.; Scheller, H.V.; Zhang, A. The NADPH-oxidase AtRbohI plays a positive role in drought-stress response in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2017, 491, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Yang, X.; Li, X.; Luo, D.; Wei, C.; Ma, J.; Zhang, Y.; Yang, J.; Zhang, X. Glutathione-dependent induction of local and systemic defense against oxidative stress by exogenous melatonin in cucumber (Cucumis sativus L.). J. Pineal Res. 2016, 60, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Luo, Z.; Dong, H.; Eneji, A.E.; Li, W. H2O2 and ABA signaling are responsible for the increased Na+ efflux and water uptake in Gossypium hirsutum L. roots in the non-saline side under non-uniform root zone salinity. J. Exp. Bot. 2016, 67, 2247–2261. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH Oxidase RBOH Functions in Rice Roots during Lysigenous Aerenchyma Formation under Oxygen-Deficient Conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Mangano, S.; Denita-Juarez, S.P.; Choi, H.-S.; Marzol, E.; Hwang, Y.; Ranocha, P.; Velasquez, S.M.; Borassi, C.; Barberini, M.L.; Aptekmann, A.A.; et al. Molecular link between auxin and ROS-mediated polar growth. Proc. Natl. Acad. Sci. USA 2017, 114, 5289–5294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voxeur, A.; Höfte, H. Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology 2016, 26, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Xia, X.-J.; Guo, X.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Yu, J.-Q. Apoplastic H2 O2 plays a critical role in axillary bud outgrowth by altering auxin and cytokinin homeostasis in tomato plants. New Phytol. 2016, 211, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, F.; Xiang, X.; Ahammed, G.J.; Wang, M.; Onac, E.; Zhou, J.; Xia, X.; Shi, K.; Yin, X.; et al. Systemic Induction of Photosynthesis via Illumination of the Shoot Apex Is Mediated Sequentially by Phytochrome, B., Auxin and Hydrogen Peroxide in Tomato. Plant Physiol. 2016, 172, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C. Leaf epinasty and auxin: A biochemical and molecular overview. Plant Sci. 2016, 253, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Gómez-Cadenas, A.; Blumwald, E.; Mittler, R. Coordinating the overall stomatal response of plants: Rapid leaf-to-leaf communication during light stress. Sci. Signal. 2018, 11, eaam9514. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Shang, Y.; Nam, K.H. Brassinosteroids modulate ABA-induced stomatal closure in Arabidopsis. J. Exp. Bot. 2016, 67, 6297–6308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.-J.; Gao, C.-J.; Song, L.-X.; Zhou, Y.-H.; Shi, K.; Yu, J.-Q. Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ. 2014, 37, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, Y.; Li, C.; Liu, Z.; Yang, N.; Pan, L.; Wu, J.; Wang, J.; Yang, J.; Lv, Y.; et al. Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol. 2018, 217, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Khokon, M.A.R.; Salam, M.A.; Jammes, F.; Ye, W.; Hossain, M.A.; Okuma, E.; Nakamura, Y.; Mori, I.C.; Kwak, J.M.; Murata, Y. MPK9 and MPK12 function in SA-induced stomatal closure in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2017, 81, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Ehonen, S.; Yarmolinsky, D.; Kollist, H.; Kangasjärvi, J. Reactive Oxygen Species, Photosynthesis and Environment in the Regulation of Stomata. Antioxid. Redox Signal. 2017, ars.2017.7455. [Google Scholar] [CrossRef]
- Ge, X.-M.; Cai, H.-L.; Lei, X.; Zhou, X.; Yue, M.; He, J.-M. Heterotrimeric G protein mediates ethylene-induced stomatal closure via hydrogen peroxide synthesis in Arabidopsis. Plant J. 2015, 82, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Qi, C.; Ren, H.; Huang, A.; Hei, S.; She, X. Ethylene mediates brassinosteroid-induced stomatal closure via Gα protein-activated hydrogen peroxide and nitric oxide production in Arabidopsis. Plant J. 2015, 82, 280–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xu, S.; Wang, Z.; He, L.; Xu, K.; Wang, G. Glucose triggers stomatal closure mediated by basal signaling through HXK1 and PYR/RCAR receptors in Arabidopsis. J. Exp. Bot. 2018, 69, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- Nazareno, A.L.; Hernandez, B.S. A mathematical model of the interaction of abscisic acid, ethylene and methyl jasmonate on stomatal closure in plants. PLoS ONE 2017, 12, e0171065. [Google Scholar] [CrossRef] [PubMed]
- Dubovskaya, L.V.; Bakakina, Y.S.; Kolesneva, E.V.; Sodel, D.L.; Mcainsh, M.R.; Hetherington, A.M.; Volotovski, I.D. cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1. New Phytol. 2011, 191, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Y.; Miao, W.; Piao, T.; Hao, Y.; Hao, F.-S. NADK2 positively modulates abscisic acid-induced stomatal closure by affecting accumulation of H2O2, Ca2+ and nitric oxide in Arabidopsis guard cells. Plant Sci. 2017, 262, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 2018, 255, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Messeder, D.; Caverzan, A.; Rauber, R.; Cunha, J.R.; Carvalho, F.E.L.; Gaeta, M.L.; da Fonseca, G.C.; Costa, J.M.; Frei, M.; Silveira, J.A.G.; et al. Thylakoidal APX modulates hydrogen peroxide content and stomatal closure in rice (Oryza sativa L.). Environ. Exp. Bot. 2018, 150, 46–56. [Google Scholar] [CrossRef]
- Mao, X.; Zheng, Y.; Xiao, K.; Wei, Y.; Zhu, Y.; Cai, Q.; Chen, L.; Xie, H.; Zhang, J. OsPRX2 contributes to stomatal closure and improves potassium deficiency tolerance in rice. Biochem. Biophys. Res. Commun. 2018, 495, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Kaya, H.; Kawarazaki, T.; Hiraoka, G.; Senzaki, E.; Michikawa, M.; Kuchitsu, K. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim. Biophys. Acta 2012, 1823, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Sirichandra, C.; Gu, D.; Hu, H.-C.; Davanture, M.; Lee, S.; Djaoui, M.; Valot, B.; Zivy, M.; Leung, J.; Merlot, S.; et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009, 583, 2982–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assmann, S.M.; Jegla, T. Guard cell sensory systems: Recent insights on stomatal responses to light, abscisic acid and CO2. Curr. Opin. Plant Biol. 2016, 33, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Scuffi, D.; Nietzel, T.; Di Fino, L.M.; Meyer, A.J.; Lamattina, L.; Schwarzländer, M.; Laxalt, A.M.; García-Mata, C. Hydrogen Sulfide Increases Production of NADPH Oxidase-Dependent Hydrogen Peroxide and Phospholipase D-Derived Phosphatidic Acid in Guard Cell Signaling. Plant Physiol. 2018, 176, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.; Feng, X.; Liu, L.; Xiong, L.; Wang, L. ALA-Induced Flavonols Accumulation in Guard Cells Is Involved in Scavenging H2O2 and Inhibiting Stomatal Closure in Arabidopsis Cotyledons. Front. Plant Sci. 2016, 7, 1713. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Liu, L.; Chen, L.; Wang, L. ALA Inhibits ABA-induced Stomatal Closure via Reducing H2O2 and Ca(2+) Levels in Guard Cells. Front. Plant Sci. 2016, 7, 482. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Romero-Puertas, M.C.; Sandalio, L.M.; Olmedilla, A. The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J. Exp. Bot. 2015, 66, 2869–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassig, R.; Gutermuth, T.; Bey, T.D.; Konrad, K.R.; Romeis, T. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J. 2014, 78, 94–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Kita, D.; Johnson, E.A.; Aggarwal, M.; Gates, L.; Wu, H.-M.; Cheung, A.Y. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 2014, 5, 3129. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol. Biochem. PPB 2016, 104, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Irfan, M.; Ghosh, S.; Chakraborty, N.; Chakraborty, S.; Datta, A. Fruit ripening mutants reveal cell metabolism and redox state during ripening. Protoplasma 2016, 253, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Hurr, B.M.; Huber, D.J.; Vallejos, C.E.; Lee, E.; Sargent, S.A. Ethylene-induced overproduction of reactive oxygen species is responsible for the development of watersoaking in immature cucumber fruit. J. Plant Physiol. 2013, 170, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ospina, L.; Moison, M.; Yoshimoto, K.; Masclaux-Daubresse, C. Autophagy, plant senescence and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar] [CrossRef] [PubMed]
- Bieker, S.; Riester, L.; Stahl, M.; Franzaring, J.; Zentgraf, U. Senescence-specific Alteration of Hydrogen Peroxide Levels in Arabidopsis thaliana and Oilseed Rape Spring Variety Brassica napus L. cv. MozartF. J. Integr. Plant Biol. 2012, 54, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, J.; Chang, Y.; Jiang, C.-Z. Comparative Transcriptomic Analysis Reveals That Ethylene/ H2O2-Mediated Hypersensitive Response and Programmed Cell Death Determine the Compatible Interaction of Sand Pear and Alternaria alternata. Front. Plant Sci. 2017, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Y.; Zhang, Z.; Wei, J. Hydrogen peroxide promotes programmed cell death and salicylic acid accumulation during the induced production of sesquiterpenes in cultured cell suspensions of Aquilaria sinensis. Funct. Plant Biol. 2015, 42, 337–346. [Google Scholar] [CrossRef]
- Zimmermann, P.; Heinlein, C.; Orendi, G.; Zentgraf, U. Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2006, 29, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Jajić, I.; Sarna, T.; Szewczyk, G.; Strzałka, K. Changes in production of reactive oxygen species in illuminated thylakoids isolated during development and senescence of barley. J. Plant Physiol. 2015, 184, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, E.; Polzien, L.; Desel, C.; Rozpadek, P.; Miszalski, Z.; Krupinska, K. Spatial patterns of senescence and development-dependent distribution of reactive oxygen species in tobacco (Nicotiana tabacum) leaves. J. Plant Physiol. 2009, 166, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross Talk between H2O2 and Interacting Signal Molecules under Plant Stress Response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of Polyamines, Abscisic Acid, Nitric Oxide and Hydrogen Peroxide under Chilling Stress in Tomato (Lycopersicon esculentum Mill.) Seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.-G.; Kong, C.-C.; Xie, Z.-H. Role of abscisic acid in strigolactone-induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol. 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Freitas, V.S.; de Souza Miranda, R.; Costa, J.H.; de Oliveira, D.F.; de Oliveira Paula, S.; de Castro Migueld, E.; Freire, R.S.; Prisco, J.T.; Gomes-Filho, E. Ethylene triggers salt tolerance in maize genotypes by modulating polyamine catabolism enzymes associated with H2O2 production. Environ. Exp. Bot. 2018, 145, 75–86. [Google Scholar] [CrossRef]
- 224 Kaur, N.; Kirat, K.; Saini, S.; Sharma, I.; Gantet, P.; Pati, P.K. Reactive oxygen species generating system and brassinosteroids are linked to salt stress adaptation mechanisms in rice. Plant Signal. Behav. 2016, 11, e1247136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-Y.; Huang, Y.-C.; Ou, S.-L. ERF73/HRE1 is involved in H2O2 production via hypoxia-inducible Rboh gene expression in hypoxia signaling. Protoplasma 2017, 254, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; He, R.J.; Xie, Q.L.; Zhao, X.H.; Deng, X.M.; He, J.B.; Song, L.; He, J.; Marchant, A.; Chen, X.-Y.; et al. ETHYLENE RESPONSE FACTOR 74 (ERF74) plays an essential role in controlling a respiratory burst oxidase homolog D (RbohD)-dependent mechanism in response to different stresses in Arabidopsis. New Phytol. 2017, 213, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jiang, F.; Cen, B.; Wen, J.; Zhou, Y.; Wu, Z. Respiratory burst oxidase homologue-dependent H2O2 and chloroplast H2O2 are essential for the maintenance of acquired thermotolerance during recovery after acclimation. Plant Cell Environ. 2018. [Google Scholar] [CrossRef] [PubMed]
- Birch, P.R.J.; Avrova, A.O.; Dellagi, A.; Lacomme, C.; Cruz, S.S.; Lyon, G.D. Programmed Cell Death in Plants in Response to Pathogen Attack. In Annual Plant. Reviews; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 184–208. [Google Scholar]
- Novák, J.; Pavlů, J.; Novák, O.; Nožková-Hlaváčková, V.; Špundová, M.; Hlavinka, J.; Koukalová, Š.; Skalák, J.; Černý, M.; Brzobohatý, B. High cytokinin levels induce a hypersensitive-like response in tobacco. Ann. Bot. 2013, 112, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nováková, M.; Šašek, V.; Trdá, L.; Krutinová, H.; Mongin, T.; Valentová, O.; Balesdent, M.-H.; Rouxel, T.; Burketová, L. L eptosphaeria maculans effector AvrLm4-7 affects salicylic acid (SA) and ethylene (ET) signalling and hydrogen peroxide (H2O2) accumulation in Brassica napus. Mol. Plant Pathol. 2016, 17, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Song, L.-X.; Xu, X.-C.; Wang, F.-N.; Wang, Y.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Zhou, J.; Yu, J.-Q. Brassinosteroids act as a positive regulator for resistance against root-knot nematode involving RESPIRATORY BURST OXIDASE HOMOLOG-dependent activation of MAPKs in tomato. Plant Cell Environ. 2018, 41, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-G.; Zhu, T.; Zou, L.-J.; Han, X.-Y.; Zhou, X.; Xi, D.-H.; Zhang, D.-W.; Lin, H.-H. Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J. 2016, 85, 478–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Chen, J.; Xiao, X.; Zhang, M.; Yun, Z.; Zeng, Y.; Xu, J.; Cheng, Y.; Deng, X. Salicylic acid treatment reduces the rot of postharvest citrus fruit by inducing the accumulation of H2O2, primary metabolites and lipophilic polymethoxylated flavones. Food Chem. 2016, 207, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ellouzi, H.; Sghayar, S.; Abdelly, C. H2O2 seed priming improves tolerance to salinity; drought and their combined effect more than mannitol in Cakile maritima when compared to Eutrema salsugineum. J. Plant Physiol. 2017, 210, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Cellular Defenses against Superoxide and Hydrogen Peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidchik, V.; Shabala, S.N.; Coutts, K.B.; Tester, M.A.; Davies, J.M. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J. Cell Sci. 2003, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Shang, Z.; Rubio, L.; Cuin, T.A.; Véry, A.-A.; Wang, A.; Mortimer, J.C.; Macpherson, N.; Coxon, K.M.; Battey, N.H.; et al. Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 2012, 24, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S. Mechanisms of cytosolic calcium elevation in plants: The role of ion channels, calcium extrusion systems and NADPH oxidase-mediated “ROS-Ca2+ Hub”. Funct. Plant Biol. 2018, 45, 9. [Google Scholar] [CrossRef]
- Sun, J.; Wang, M.-J.; Ding, M.-Q.; Deng, S.-R.; Liu, M.-Q.; Lu, C.-F.; Zhou, X.-Y.; Shen, X.; Zheng, X.-J.; Zhang, Z.-K.; et al. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 2010, 33, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, T.; Lehotai, N.; Strand, Å. The role of retrograde signals during plant stress responses. J. Exp. Bot. 2018, 69, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the crossroad of abiotic stress signalling pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, A.; Kelesidis, G.A.; Zuercher, S.; Krumeich, F.; Bolisetty, S.; Mezzenga, R.; Leroux, J.-C.; Sotiriou, G.A. Enzyme-Mimetic Antioxidant Luminescent Nanoparticles for Highly Sensitive Hydrogen Peroxide Biosensing. ACS Nano 2017, 11, 12210–12218. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.J.; Gupta, A.; Barkam, S.; Saraf, S.; Das, S.; Cho, H.J.; Seal, S. Picomolar Detection of Hydrogen Peroxide using Enzyme-free Inorganic Nanoparticle-based Sensor. Sci. Rep. 2017, 7, 1324. [Google Scholar] [CrossRef] [PubMed]
- Shabrangy, A.; Roustan, V.; Reipert, S.; Weidinger, M.; Roustan, P.-J.; Stoger, E.; Weckwerth, W.; Ibl, V. Using RT-qPCR, Proteomics and Microscopy to Unravel the Spatio-Temporal Expression and Subcellular Localization of Hordoindolines Across Development in Barley Endosperm. Front. Plant Sci. 2018, 9, 775. [Google Scholar] [CrossRef] [PubMed]


| AGI | Protein Name (UniProt) | Relative Protein Abundance | ||||||
|---|---|---|---|---|---|---|---|---|
| Auxin | Abscisic Acid | Brassinosteroid | Cytokinin | Salicylic Acid | Jasmonate/Oxylipins | Strigolactone | ||
| AT1G05260 | Peroxidase 3 | down [121] | ||||||
| AT1G06290 | Acyl-coenzyme A oxidase 3 | up [122] | ||||||
| AT1G07890 | L-Ascorbate peroxidase 1 | down [121] | down [30,123,124] | up [125,126] | ||||
| AT1G08830 | Superoxide dismutase [Cu-Zn] 1 | up [127] | ||||||
| AT1G20620 | Catalase-3 | up [128] | down [129] | up [130] | up [130] | |||
| AT1G20630 | Catalase-1 | up [131] | ||||||
| AT1G31710 | Amine oxidase | down [121] | ||||||
| AT1G44446 | Chlorophyllide a oxygenase | down [132] | ||||||
| AT1G65980 | Peroxiredoxin-2B | down [121] | up [126] | |||||
| AT1G71695 | Peroxidase 12 | down [121] | down [132] | |||||
| AT1G77490 | L-Ascorbate peroxidase T | up [133] | ||||||
| AT2G18150 | Peroxidase 15 | up [127] | ||||||
| AT2G22420 | Peroxidase 17 | up [127] | ||||||
| AT2G26230 | Uricase | down [121] | ||||||
| AT2G28190 | Superoxide dismutase [Cu-Zn] 2 | up [134] | ||||||
| AT2G30490 | Trans-cinnamate 4-monooxygenase | up [131] | ||||||
| AT2G43350 | Probable glutathione peroxidase 3 | down [121] | ||||||
| AT3G06050 | Peroxiredoxin-2F | up [134] | ||||||
| AT3G10920 | Superoxide dismutase [Mn] 1 | down [135] | up [136] | down [126] | ||||
| AT3G11630 | 2-Cys peroxiredoxin BAS1 | up [30,129] | up [125] | |||||
| AT3G14415 | (S)-2-hydroxy-acid oxidase | down [125] | up [130] | up [125,130] | ||||
| AT3G14420 | (S)-2-hydroxy-acid oxidase GLO1 | up [30] | up [130] | up [126,130] | ||||
| AT3G26060 | Peroxiredoxin Q, chloroplastic | up [134] | ||||||
| AT3G32980 | Peroxidase 32 | down [30] | up [127] | |||||
| AT3G49120 | Peroxidase 34 | up [128,131] | down [30] | up [127] | ||||
| AT3G56350 | Superoxide dismutase [Mn] 2 | up [137] | ||||||
| AT4G08390 | L-Ascorbate peroxidase S | up [126] | down [30] | |||||
| AT4G08770 | Peroxidase 37 | up [127] | ||||||
| AT4G08780 | Peroxidase 38 | up [127] | ||||||
| AT4G15760 | Monooxygenase 1 | up [137] | ||||||
| AT4G16760 | Acyl-coenzyme A oxidase 1 | up [133] | up [122] | |||||
| AT4G25100 | Superoxide dismutase [Fe] 1 | up [127] | up [125] | |||||
| AT4G35000 | L-Ascorbate peroxidase 3 | down [30] | ||||||
| AT4G35090 | Catalase-2 | up [131] | up/down [30,123] | up [130] | up [130] | |||
| AT4G36430 | Peroxidase 49 | up [127] | ||||||
| AT5G06290 | 2-Cys peroxiredoxin BAS1-like | up [126] | ||||||
| AT5G14220 | Protoporphyrinogen oxidase 2 | up [132] | up [132] | |||||
| AT5G17820 | Peroxidase 57 | up [128] | ||||||
| AT5G18100 | Superoxide dismutase [Cu-Zn] 3 | up [127] | ||||||
| AT5G23310 | Superoxide dismutase [Fe] 3 | down [138] | down [122] | |||||
| AT5G49970 | PYRIDOXINE/PYRIDOXAMINE 5′-PHOSPHATE OXIDASE 1 | up [122] | ||||||
| AT5G51100 | Superoxide dismutase [Fe] 2 | up [139] | ||||||
| AT5G64120 | Peroxidase 71 | down [131] | up [122] | down [137] | ||||
| AT5G65110 | Acyl-coenzyme A oxidase 2 | down [132] | ||||||
| Nutrient Stress (142) | Temperature Stress (43) | Drought Stress (13) | Light Signalling (27) | Abscisic Acid Metabolism (16) | Auxin Metabolism (31) | Brassinosteroid Metabolism (13) | Cytokinin Metabolism (37) | Ethylene Metabolism (12) | Gibberellin Metabolism (23) | Jasmonate Metabolism (17) | Salicylic Acid Metabolism (9) | Strigolactone Metabolism (3) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine/polyamine oxidase (15) | 11/40 | 4/6 | 1/1 | 7/16 | 6/5 | 5/6 | 5/5 | 10/15 | 5/4 | 4/7 | 3/5 | 2/1 | 0/0 |
| Respiratory burst oxidase (10) | 10/58 | 6/6 | 4/1 | 9/16 | 5/3 | 7/14 | 8/8 | 9/21 | 4/4 | 8/6 | 3/2 | 4/4 | 4/3 |
| Superoxide dismutase (8) | 7/46 | 5/5 | 0/0 | 4/13 | 5/2 | 5/9 | 4/6 | 5/15 | 2/3 | 5/10 | 1/3 | 2/2 | 0/0 |
| L-Gulonolactone oxidase (7) | 7/31 | 2/2 | 2/1 | 4/5 | 4/5 | 4/7 | 2/1 | 7/10 | 3/3 | 3/5 | 3/4 | 3/2 | 2/2 |
| Acyl-coenzyme A oxidase (7) | 5/19 | 4/6 | 4/2 | 4/14 | 4/3 | 6/9 | 4/2 | 5/11 | 0/0 | 5/7 | 6/6 | 1/1 | 2/2 |
| Glycolate oxidase (5) | 5/10 | 2/1 | 0/0 | 5/8 | 0/0 | 3/3 | 4/2 | 3/4 | 0/0 | 2/1 | 3/2 | 0/0 | 3/1 |
| Aldehyde/acetaldehyde oxidase (5) | 4/36 | 2/1 | 2/1 | 4/9 | 3/3 | 5/8 | 4/4 | 4/13 | 1/1 | 3/7 | 1/2 | 3/3 | 1/2 |
| Long-chain-alcohol oxidase (4) | 3/18 | 3/4 | 0/0 | 2/7 | 2/2 | 3/5 | 1/1 | 3/6 | 1/1 | 1/1 | 1/2 | 2/2 | 0/0 |
| Sulfhydryl oxidase (3) | 3/25 | 3/5 | 0/0 | 3/13 | 3/2 | 3/8 | 3/3 | 3/5 | 2/2 | 3/6 | 3/3 | 2/1 | 1/1 |
| Protoporphyrinogen oxidase (2) | 2/21 | 2/1 | 0/0 | 2/5 | 2/2 | 2/6 | 2/3 | 2/6 | 2/1 | 2/3 | 0/0 | 1/2 | 0/0 |
| Pyridoxal 5′-phosphate synthase (2) | 2/28 | 2/3 | 0/0 | 2/9 | 2/2 | 2/7 | 2/3 | 2/8 | 2/2 | 2/5 | 1/1 | 2/2 | 1/1 |
| L-Aspartate oxidase (1) | 1/5 | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 0/0 | 0/0 | 1/1 |
| Sarcosine oxidase (1) | 1/1 | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Uricase (1) | 1/6 | 1/1 | 1/1 | 1/3 | 1/1 | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 |
| Sulphite oxidase (1) | 1/12 | 1/3 | 0/0 | 1/9 | 1/1 | 1/5 | 1/1 | 1/4 | 1/1 | 1/3 | 1/1 | 1/1 | 0/0 |
| Peroxidase (73) | 53/106 | 21/9 | 9/2 | 33/25 | 21/7 | 35/21 | 29/13 | 43/30 | 21/6 | 27/15 | 17/8 | 18/7 | 10/3 |
| Peroxiredoxin (10) | 8/43 | 4/7 | 1/1 | 5/14 | 5/3 | 3/7 | 3/5 | 6/15 | 4/4 | 7/12 | 2/4 | 4/3 | 0/0 |
| L-Ascorbate peroxidase (7) | 6/44 | 6/7 | 2/2 | 6/16 | 3/2 | 4/10 | 5/7 | 6/16 | 3/4 | 6/9 | 4/3 | 3/2 | 2/2 |
| Glutathione peroxidase (6) | 5/26 | 3/6 | 0/0 | 3/10 | 1/1 | 5/8 | 3/3 | 3/6 | 3/2 | 3/3 | 1/4 | 1/1 | 2/2 |
| Catalase (3) | 3/13 | 2/2 | 1/1 | 2/13 | 1/1 | 3/5 | 2/3 | 3/8 | 0/0 | 3/3 | 2/4 | 0/0 | 2/1 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. https://doi.org/10.3390/ijms19092812
Černý M, Habánová H, Berka M, Luklová M, Brzobohatý B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. International Journal of Molecular Sciences. 2018; 19(9):2812. https://doi.org/10.3390/ijms19092812
Chicago/Turabian StyleČerný, Martin, Hana Habánová, Miroslav Berka, Markéta Luklová, and Břetislav Brzobohatý. 2018. "Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks" International Journal of Molecular Sciences 19, no. 9: 2812. https://doi.org/10.3390/ijms19092812