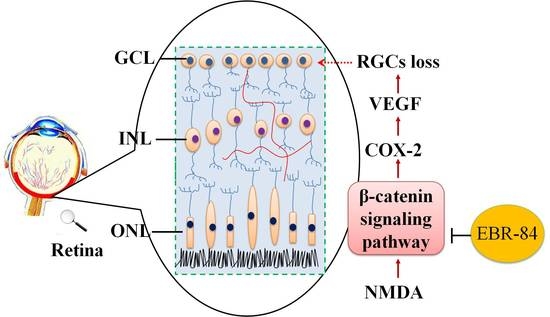
Extract of the Blood Circulation-Promoting Recipe-84 Can Protect Rat Retinas by Inhibiting the β-Catenin Signaling Pathway
Abstract
:1. Introduction
2. Results
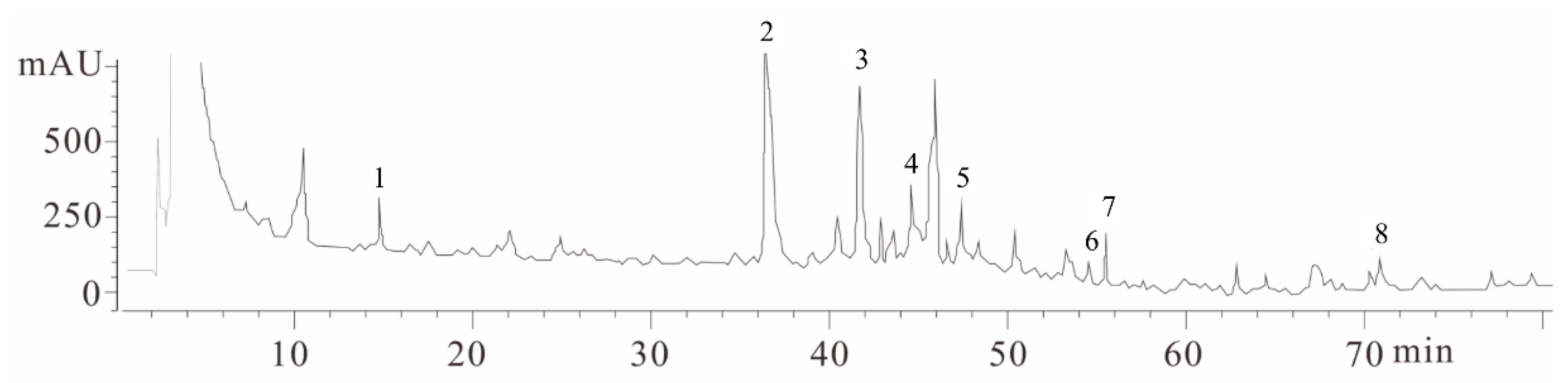
2.1. Fingerprint Chromatogram of EBR-84 by High Performance Liquid Chromatography (HPLC)
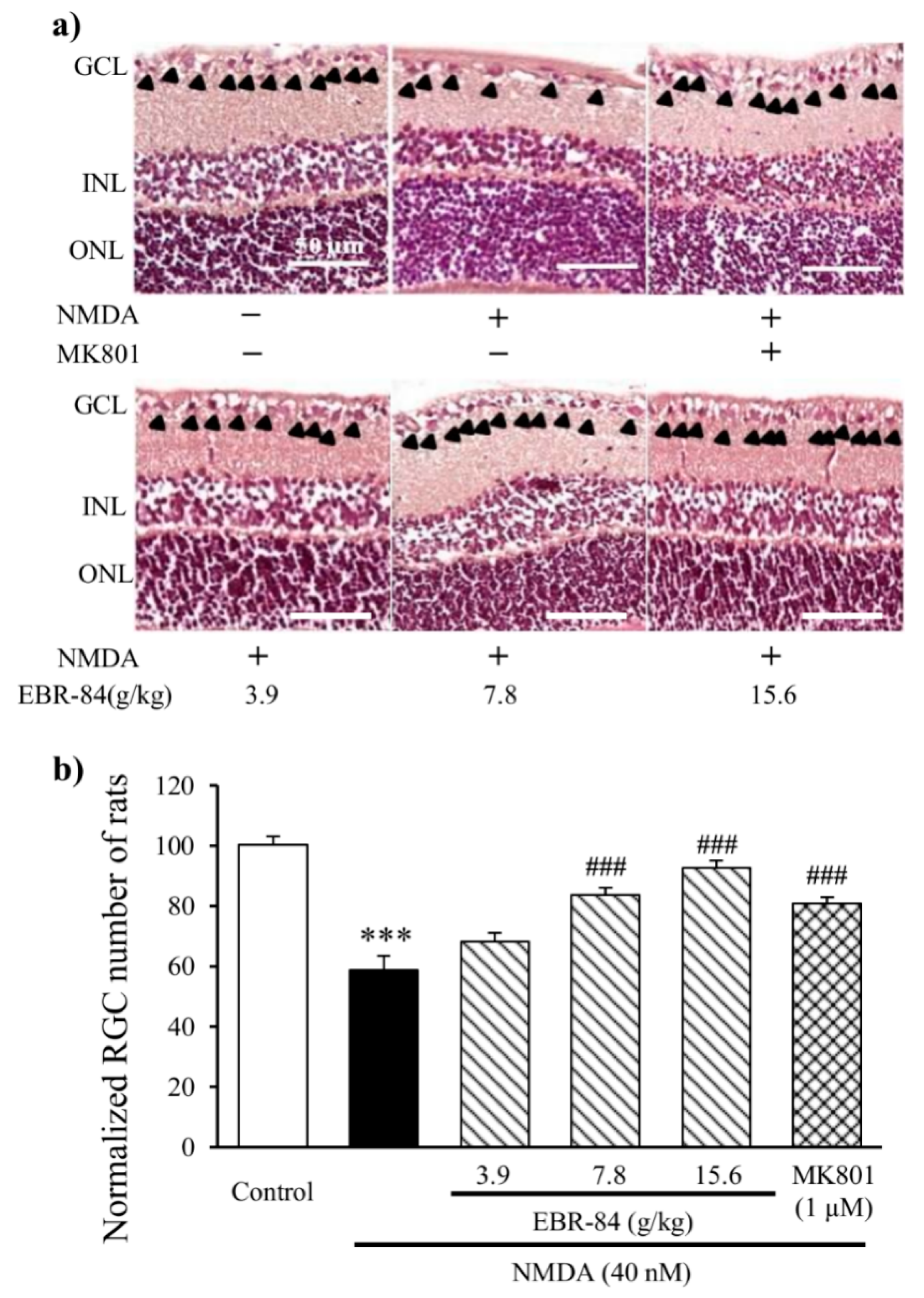
2.2. EBR-84 Retarded the RGC Loss of the NMDA-Treated Rats
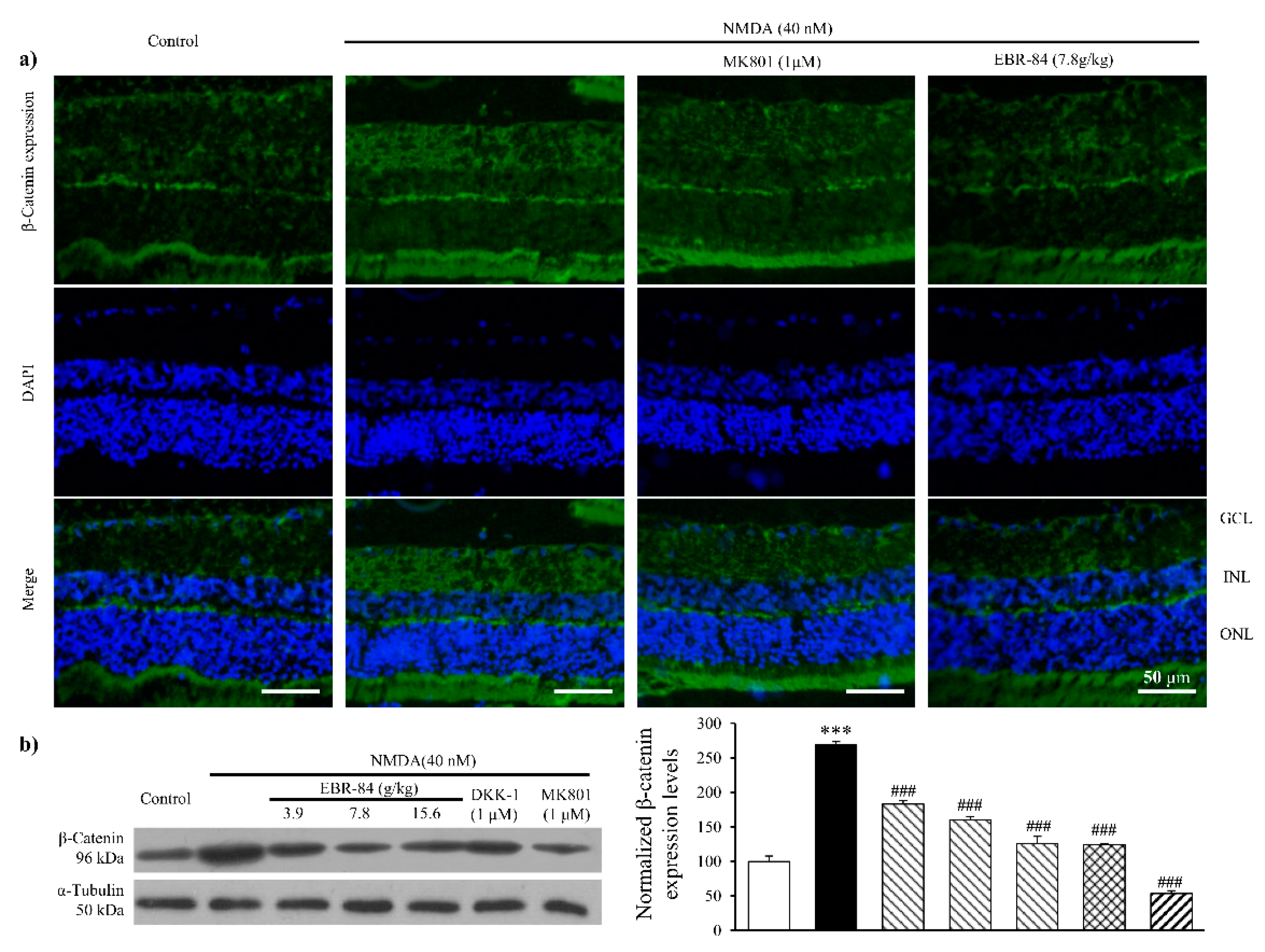
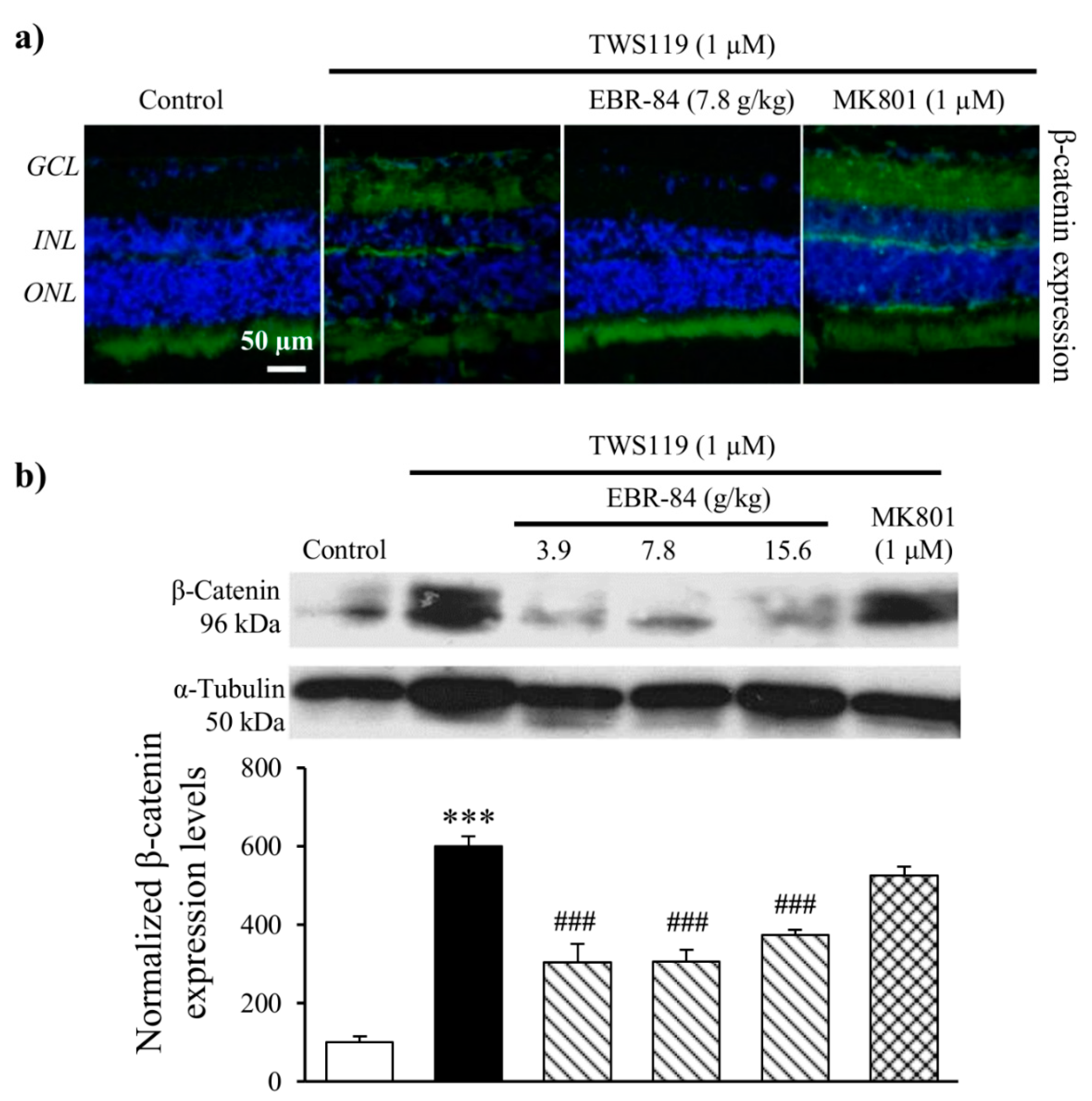
2.3. EBR-84 Decreased Retinal β-Catenin Expression of NMDA-Treated Rats
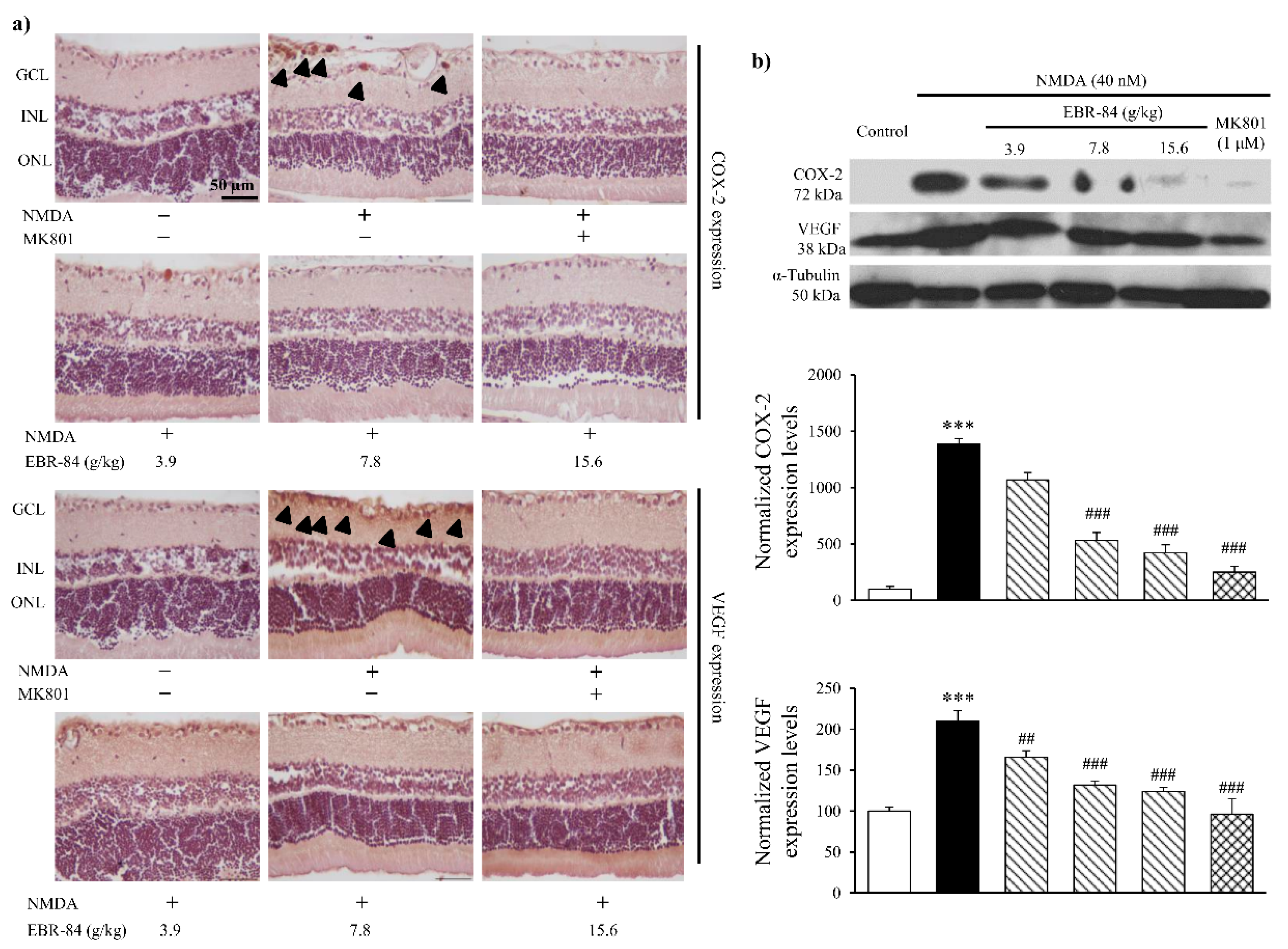
2.4. EBR-84 Downregulated COX-2 and VEGF Expression in NMDA-Treated Retinas
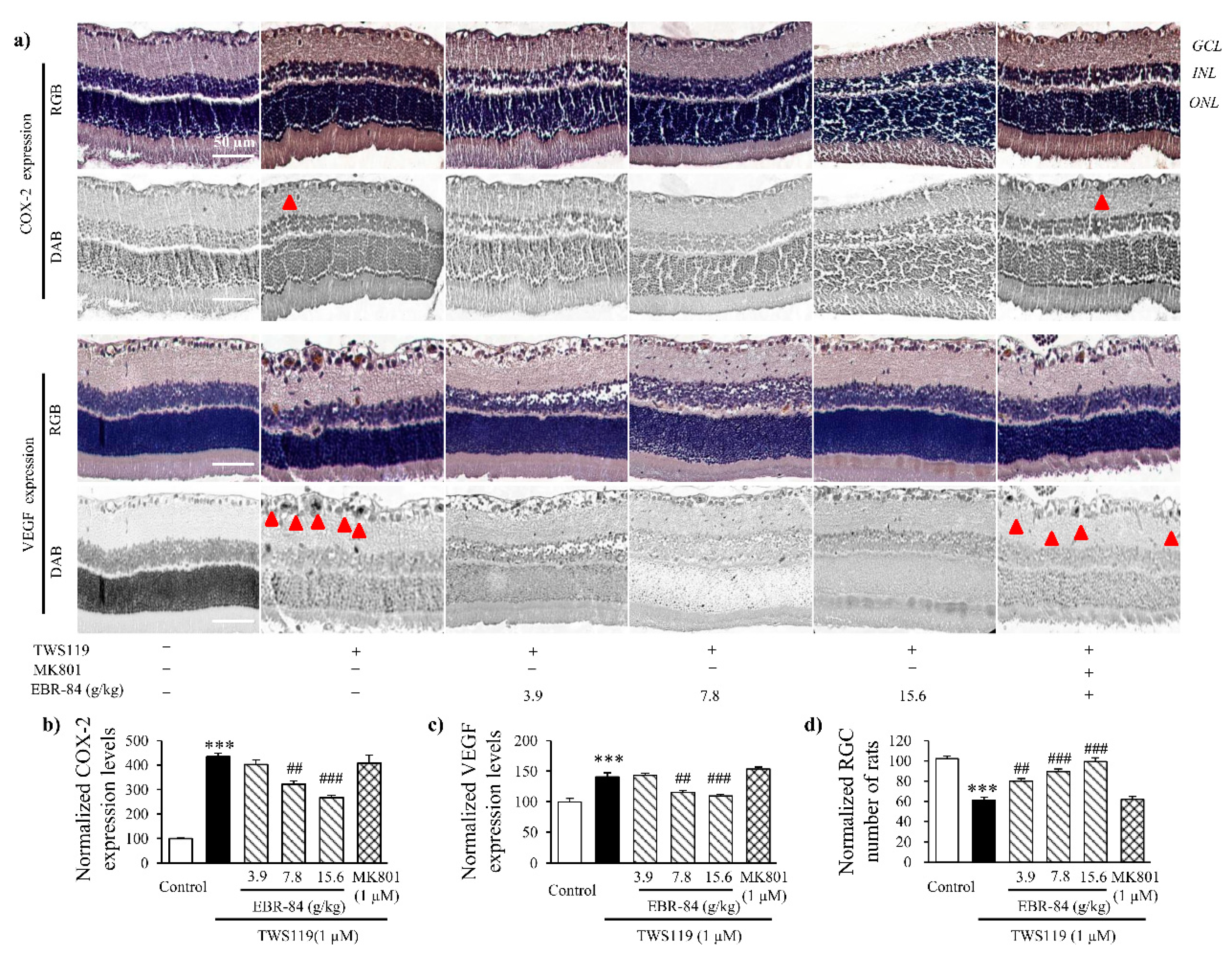
2.5. Effects of EBR on Retinas of TWS119-Treated Rats
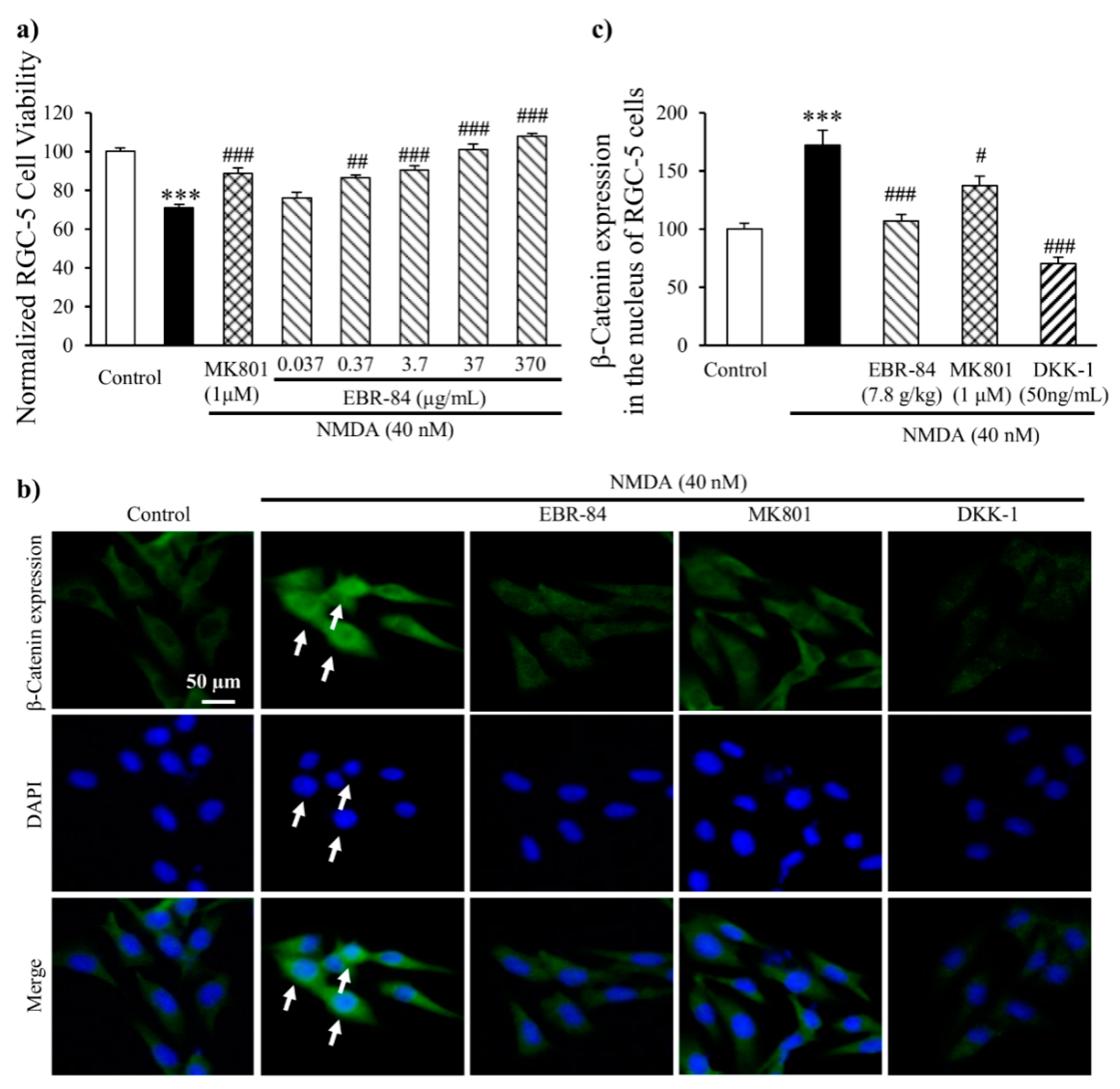
2.6. EBR-84 Increased the Viability of NMDA-Treated RGC-5 Cells
2.7. EBR-84 Depressed the β-Catenin Expression in RGC-5 Cells
3. Discussion
4. Materials and Methods
4.1. Drug Preparations
4.2. Fingerprint Chromatogram of RIE by HPLC
4.3. Animal Grouping and Drug Treatment
4.4. H&E Staining and Immunohistochemical Analysis
4.5. Western Blot Assay
4.6. Cell Culture and Viability Assay
4.7. Immunocytochemical Staining for β-Catenin
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| COX-2 | Cyclooxygenase-2 |
| DKK-1 | Dickkopf-related protein 1 |
| EBR-84 | Extract of the Blood Circulation-Promoting Recipe |
| FGF | Fibroblast growth factor |
| GSK-3β | Glycogen synthase kinase-3β |
| GCL | Ganglion cell layer |
| H&E | Hematoxylin and eosin |
| HPLC | High performance liquid chromatography |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NMDA | N-methyl-d-aspartic acid |
| NMDAR | N-methyl-d-aspartic acid receptor |
| RGCs | Retinal ganglion cells |
| SEM | Standard error of the mean |
| TGF-β | Transforming growth factor-β |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless/Integrated |
References
- Ueda, K.; Nakahara, T.; Hoshino, M.; Mori, A.; Sakamoto, K.; Ishii, K. Retinal blood vessels are damaged in a rat model of NMDA-induced retinal degeneration. Neurosci. Lett. 2010, 485, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Asano, D.; Kondo, R.; Mori, A.; Arima, S.; Ushikubo, H.; Sakamoto, K.; Nagamitsu, T.; Ishii, K.; Nakahara, T. Retinal neuronal cell loss prevents abnormal retinal vascular growth in a rat model of retinopathy of prematurity. Exp. Eye Res. 2018, 168, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Zhang, W.K.; Tian, H.; Li, X.J.; Liu, M.; Li, Y.S.; Tang, H.B. The involvement of β-catenin/COX-2/VEGF axis in NMDA-caused retinopathy. J. Ophthalmol. 2017, 2017, 9760501. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, G.; Wood, J.P.; Casson, R.J. Expression of inducible heat shock proteins Hsp27 and Hsp70 in the visual pathway of rats subjected to various models of retinal ganglion cell injury. PLoS ONE 2014, 9, e114838. [Google Scholar] [CrossRef] [PubMed]
- Buldyrev, I.; Puthussery, T.; Taylor, W.R. Synaptic pathways that shape the excitatory drive in an off retinal ganglion cell. J. Neurophysiol. 2012, 107, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Homann, J.; Freed, M.A. A mammalian retinal ganglion cell implements a neuronal computation that maximizes the SNR of its postsynaptic currents. J. Neurosci. 2017, 37, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Nakahara, T.; Mori, A.; Sakamoto, K.; Ishii, K. Protective effects of TGF-β inhibitors in a rat model of NMDA-induced retinal degeneration. Eur. J. Pharmacol. 2013, 699, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Dong, L.D.; Chen, J.; Hu, X.C.; Yang, X.L.; Wang, Z. Involvement of calpain/p35-p25/Cdk5/NMDAR signaling pathway in glutamate-induced neurotoxicity in cultured rat retinal neurons. PLoS ONE 2012, 7, e42318. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Z.; Li, B.; Li, Y.C.; Yang, X.L.; Zhang, W.; Zhong, L.; Tang, S.J. Activation of NMDA receptors upregulates a disintegrin and metalloproteinase 10 via a Wnt/MAPK signaling pathway. J. Neurosci. 2012, 32, 3910–3916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, K.K.; Ma, J.X. Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the Wnt/beta-catenin pathway. Diabetes 2010, 59, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Nosho, K.; Ohnishi, M.; Suemoto, Y.; Kirkner, G.J.; Dehari, R.; Meyerhardt, J.A.; Fuchs, C.S.; Ogino, S. Correlation of β-catenin localization with cyclooxygenase-2 expression and CpG island methylator phenotype (CIMP) in colorectal cancer. Neoplasia 2007, 9, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Nunez, F.; Bravo, S.; Cruzat, F.; Montecino, M.; De Ferrari, G.V. Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PLoS ONE 2011, 6, e18562. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.N.; Schwartz, D.R.; Vonica, A.; Brivanlou, A.H.; Cho, K.R.; Varmus, H.E. FGF-20 and DKK1 are transcriptional targets of β-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005, 24, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Mi, C.; Zhang, X.; Li, Y. Relationship between expression of β-catenin and VEGFS(VEGFA,VEGF-C),VEGF receptors-2(VEGFR-2) in medulloblastoma. Chin. J. Cancer Res. 2008, 20, 44–48. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Li, Y.; Song, Y.; Lan, L.; Tang, H. Neuroprotective effect of compound sanqi extract on rat retinal damage induced by N-methyl-d-aspartate. J. South-Cent. Univ. Nat. (Nat. Sci. Ed.) 2011, 30, 54–58. [Google Scholar]
- Xi, X.H.; Jiang, D.Y.; Tang, C.Z.; Tan, J.Q.; Nie, A.G. Effect of Panax notoginseng saponins combined isovolumic haemodilution on the retinal microcirculation of patients with retinal vein occlusion. Hunan Yi Ke Da Xue Xue Bao 2000, 25, 376–378. [Google Scholar] [PubMed]
- Kikuchi, M.; Tenneti, L.; Lipton, S.A. Role of p38 mitogen-activated protein kinase in axotomy-induced apoptosis of rat retinal ganglion cells. J. Neurosci. 2000, 20, 5037–5044. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.W.; Ren, Y.; Pei, J.Z.; Ren, B.C.; He, Y. Pigment epithelium-derived factor protects retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Int. J. Ophthalmol. 2017, 10, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Liu, M.; Nakata, Y.; Tang, H.B. β-catenin accumulation in nuclei of hepatocellular carcinoma cells up-regulates glutathione-s-transferase M3 mRNA. World J. Gastroenterol. 2011, 17, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Hanley, P.J.; Liu, E.; Chakraborty, R.; Bollard, C.; Shpall, E.; Rooney, C.; Savoldo, B.; Rodgers, J.; Dotti, G. Activation of Wnt signaling arrests effector differentiation in human peripheral and cord blood-derived T lymphocytes. J. Immunol. 2011, 187, 5221–5232. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Kuang, H.Y.; Fu, Z.; Gao, X.Y.; Liu, Y.; Deng, W. Exenatide prevents high-glucose-induced damage of retinal ganglion cells through a mitochondrial mechanism. Neurochem. Int. 2012, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y.; Reh, T.A. Activation of BMP-Smad1/5/8 signaling promotes survival of retinal ganglion cells after damage in vivo. PLoS ONE 2012, 7, e38690. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Zhou, T.; Zhou, K.K.; Mott, R.; Wu, M.; Boulton, M.; Lyons, T.J.; Gao, G.; Ma, J.X. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am. J. Pathol. 2009, 175, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Ooto, S.; Akagi, T.; Kageyama, R.; Akita, J.; Mandai, M.; Honda, Y.; Takahashi, M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. USA 2004, 101, 13654–13659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sennlaub, F.; Valamanesh, F.; Vazquez-Tello, A.; El-Asrar, A.M.; Checchin, D.; Brault, S.; Gobeil, F.; Beauchamp, M.H.; Mwaikambo, B.; Courtois, Y.; et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation 2003, 108, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yanni, S.E.; McCollum, G.W.; Penn, J.S. Genetic deletion of cox-2 diminishes vegf production in mouse retinal muller cells. Exp. Eye Res. 2010, 91, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zuo, S.; Wang, Y.; Shi, H.; Yan, S.; Chen, D.; Xiao, B.; Zhang, J.; Gong, Y.; Shi, M.; et al. Thromboxane governs the differentiation of adipose-derived stromal cells toward endothelial cells in vitro and in vivo. Circ. Res. 2016, 118, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P. Vascular endothelial growth factor. 20th-century mechanisms, 21st-century therapies. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1647–1652. [Google Scholar]
- Achen, M.G.; Stacker, S.A. Vascular endothelial growth factor-D: Signaling mechanisms, biology, and clinical relevance. Growth Factors 2012, 30, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Cho, C.S.; Kim, J.H.; Jun, H.O.; Kim, J.H. Animal models of diabetic retinopathy: Doors to investigate pathogenesis and potential therapeutics. J. Biomed. Sci. 2013, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yanagi, Y.; Tamaki, Y.; Uchida, S.; Muranaka, K. Cox-2-selective inhibitor, etodolac, suppresses choroidal neovascularization in a mice model. Biochem. Biophys. Res. Commun. 2004, 325, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Sin, M.; Chrapek, O.; Karhanova, M.; Pracharova, Z.; Langova, K.; Rehak, J. Progression of macular atrophy after PDT combined with the COX-2 inhibitor Nabumetone in the treatment of neovascular ARMD. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2014, 158, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.S.; Xi, Y.; Li, X.J.; Leng, C.L.; Jia, M.M.; Zhang, W.K.; Tang, H.B. Up-regulation of the biosynthesis and release of substance P through Wnt/β-catenin signaling pathway in rat dorsal root ganglion cells. PLoS ONE 2015, 10, e0129701. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Li, B.; Wang, Y.; Shan, A.; Shen, W.; Yuan, L.; Zhong, S. Effects of salvianolic acid b on proliferation, neurite outgrowth and differentiation of neural stem cells derived from the cerebral cortex of embryonic mice. Sci. China Life Sci. 2010, 53, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Horie, Y.; Fan, J.Y.; Sun, K.; Guo, J.; Miura, S.; Hibi, T. Potential of 3,4-dihydroxy-phenyl lactic acid for ameliorating ischemia-reperfusion-induced microvascular disturbance in rat mesentery. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G36–G44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, R.; Wang, T.; Jiang, N.; Liu, T.; Du, Y.; Li, C.; Zhang, L.; Fu, F. Protective effects of Danshensu on liver injury induced by omethoate in rats. Toxicol. Mech. Methods 2010, 20, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Lipton, S.A. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog. Brain Res. 2008, 173, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.U.; Tian, H.; Yu-Sang, L.I.; Yao, G.Q.; Tang, H.B.; Feng, L.J.; Liu, M. Neuroprotective mechanism of cyclooxygenase-2 inhibitor on rat retinal ganglion cell layer neurons damage induced by N-methyl-d-aspartate. Recent Adv. Ophthalmol. 2014, 34, 301–305. [Google Scholar]
- Witte, M.D.; Kallemeijn, W.W.; Aten, J.; Li, K.Y.; Strijland, A.; Donker-Koopman, W.E.; van den Nieuwendijk, A.M.; Bleijlevens, B.; Kramer, G.; Florea, B.I.; et al. Ultrasensitive in situ visualization of active glucocerebrosidase molecules. Nat. Chem. Biol. 2010, 6, 907–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.S.; Wang, J.X.; Jia, M.M.; Liu, M.; Li, X.J.; Tang, H.B. Dragon’s blood inhibits chronic inflammatory and neuropathic pain responses by blocking the synthesis and release of substance p in rats. J. Pharmacol. Sci. 2012, 118, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hu, Y.; Chen, Y.; Zhou, K.K.; Zhang, B.; Gao, G.; Ma, J.X. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Jia, M.M.; Li, Y.S.; Yang, Y.L.; Mao, X.Q.; Tang, H.B. Involvement of substance p/neurokinin-1 receptor in the analgesic and anticancer activities of minimally toxic fraction from the traditional Chinese medicine Liu-Shen-Wan in vitro. Biol. Pharm. Bull. 2014, 37, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.B.; Li, Y.S.; Arihiro, K.; Nakata, Y. Activation of the neurokinin-1 receptor by substance P triggers the release of substance P from cultured adult rat dorsal root ganglion neurons. Mol. Pain 2007, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wu, C.J.; Chu, G.C.; Xiao, Y.; Ho, D.; Zhang, J.; Perry, S.R.; Labrot, E.S.; Wu, X.; Lis, R.; et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature 2011, 470, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| NMDA Experimental Group | ||||
| Groups | NMDA (40 nM) | EBR-84 (g/kg) | MK801 (1 µM) | Number of Rats |
| Control group | − | − | − | 5 |
| NMDA group | + | − | − | 5 |
| EBR-84 groups | + | 3.9 | − | 5 |
| + | 7.8 | − | 5 | |
| + | 15.6 | − | 5 | |
| MK801 group | + | − | + | 5 |
| TWS119 Experimental Group | ||||
| Groups | TWS119 (1 µM) | EBR-84 (g/kg) | MK801 (1 µM) | Number of Rats |
| Control group | − | − | − | 5 |
| TWS119 group | + | − | − | 5 |
| EBR-84 groups | + | 3.9 | − | 5 |
| + | 7.8 | − | 5 | |
| + | 15.6 | − | 5 | |
| MK801 group | + | − | + | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Q.-F.; Liu, M.; Tian, G.-H.; Chen, J.; Li, Y.-S. Extract of the Blood Circulation-Promoting Recipe-84 Can Protect Rat Retinas by Inhibiting the β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2712. https://doi.org/10.3390/ijms19092712
Qin Q-F, Liu M, Tian G-H, Chen J, Li Y-S. Extract of the Blood Circulation-Promoting Recipe-84 Can Protect Rat Retinas by Inhibiting the β-Catenin Signaling Pathway. International Journal of Molecular Sciences. 2018; 19(9):2712. https://doi.org/10.3390/ijms19092712
Chicago/Turabian StyleQin, Qiu-Fang, Min Liu, Gui-Hua Tian, Jian Chen, and Yu-Sang Li. 2018. "Extract of the Blood Circulation-Promoting Recipe-84 Can Protect Rat Retinas by Inhibiting the β-Catenin Signaling Pathway" International Journal of Molecular Sciences 19, no. 9: 2712. https://doi.org/10.3390/ijms19092712