CaSK23, a Putative GSK3/SHAGGY-Like Kinase of Capsicum annuum, Acts as a Negative Regulator of Pepper’s Response to Ralstonia solanacearum Attack
Abstract
:1. Introduction
2. Results
2.1. Cloning and Sequence Analysis of CaSK23
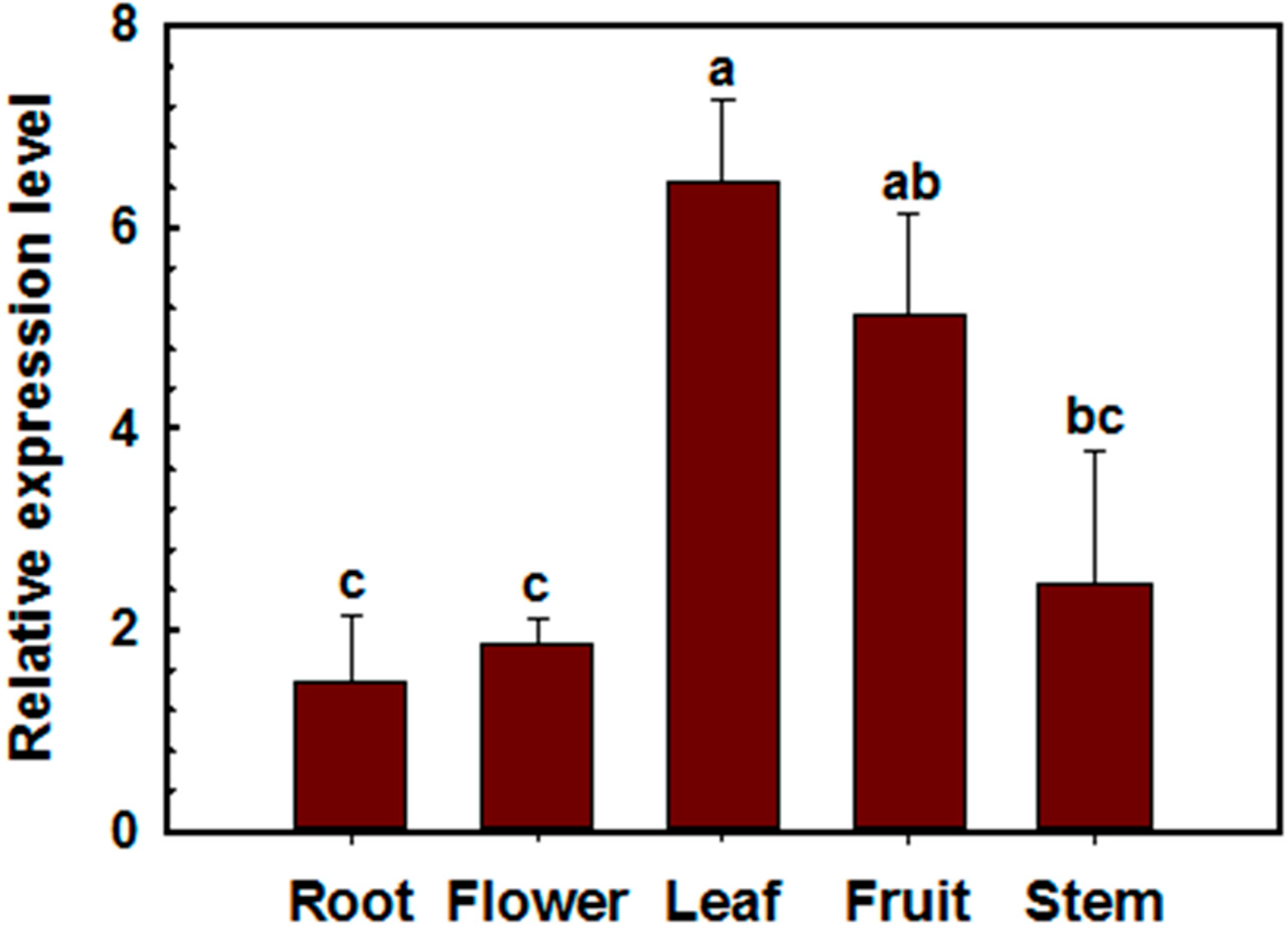
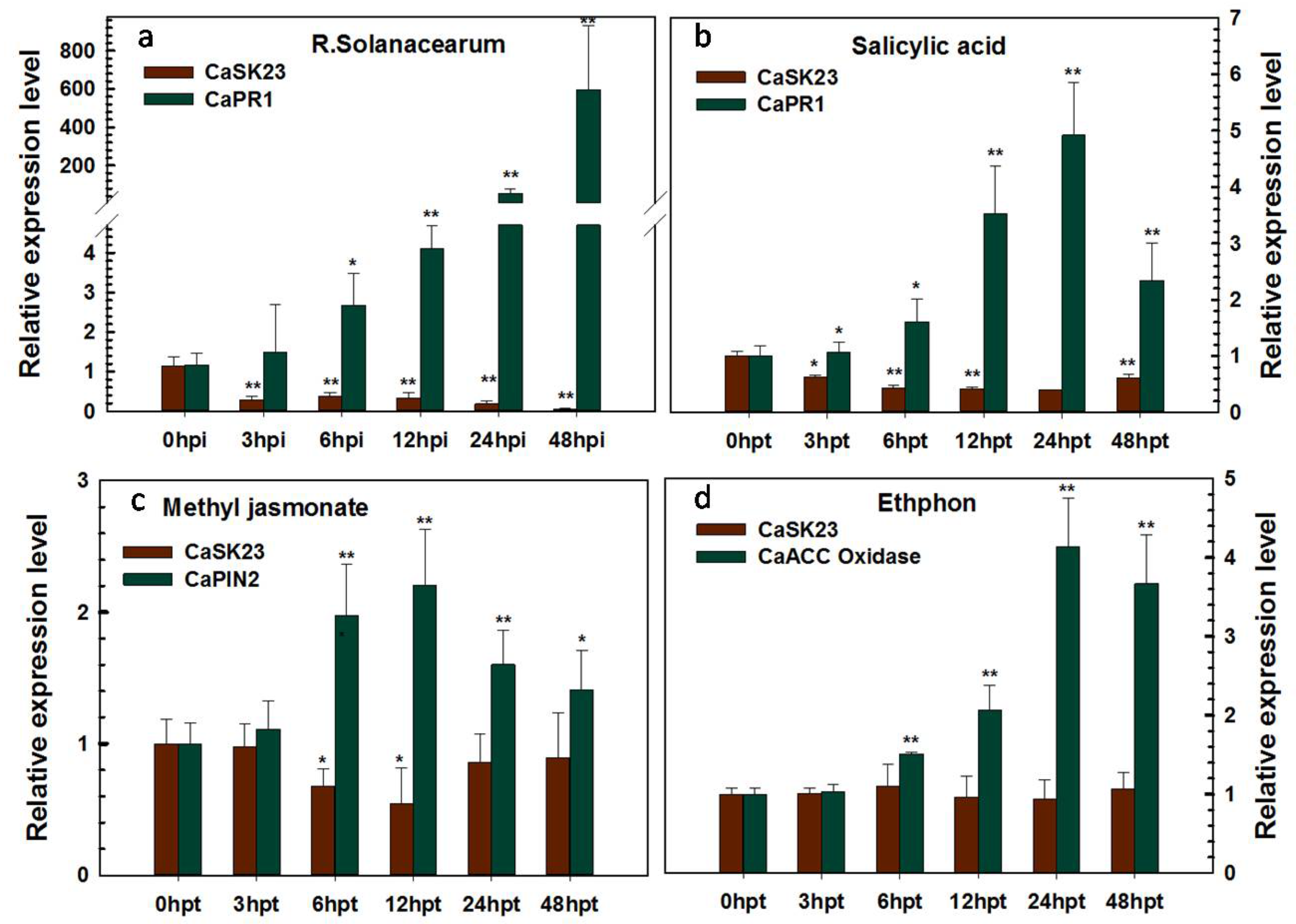
2.2. Transcriptional Expression of CaSK23 in Pepper Organs and Leaves after R. solanacearum or Exogenoushormones
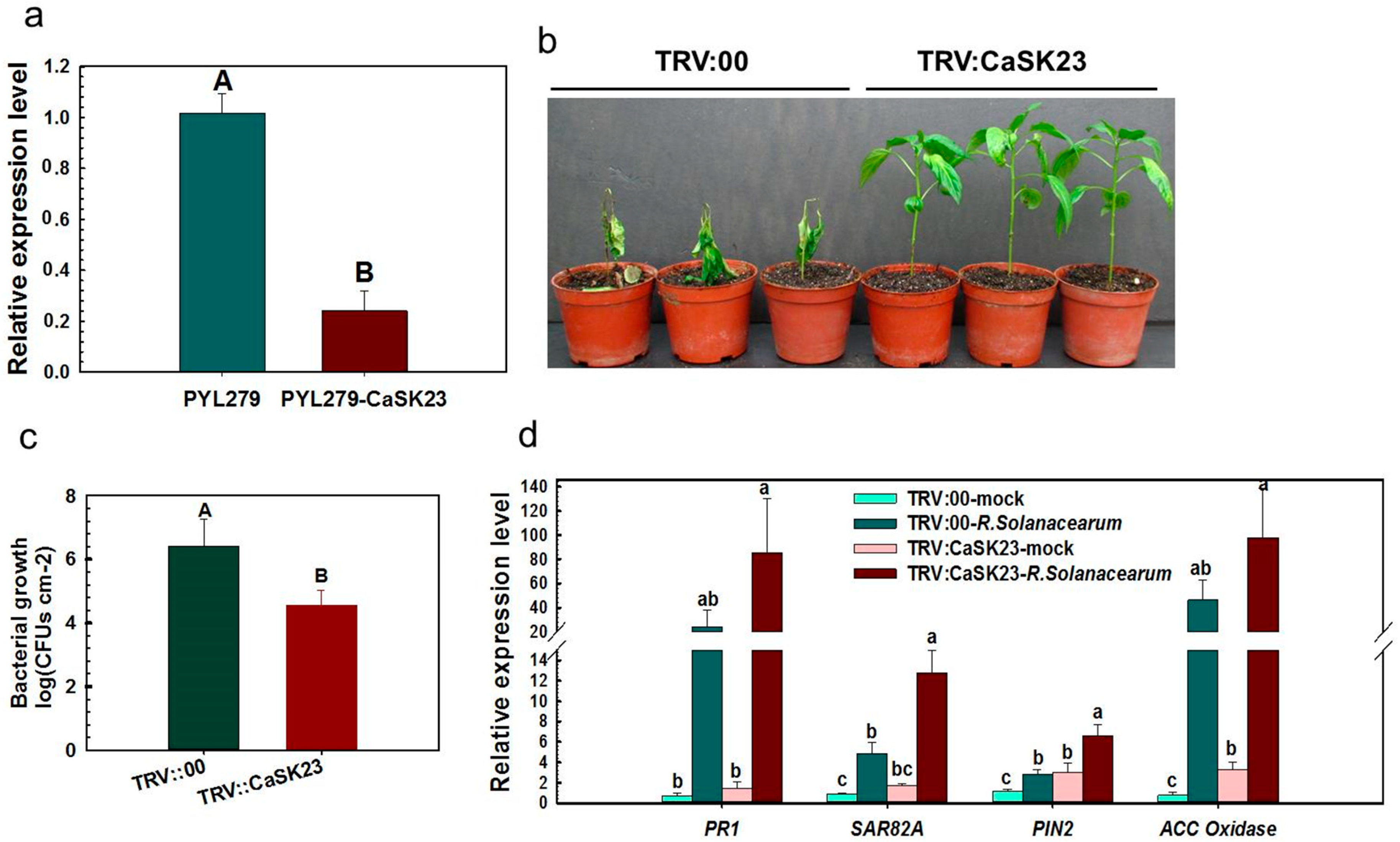
2.3. Silencing of CaSK23 by VIGS Decreased Susceptibility of Pepper Plants to R. solanacearum
2.4. Ectopic Overexpression of CaSK23 Increased Susceptibility of Transgenic Tobacco Plants to R. solanacearum
2.5. Transient Expression of CaSK23 Suppressed Expression of Defense-Associated Marker Genes in Pepper Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Isolation of CaSK23 and Its Sequence Analysis
4.3. Vector Construction
4.4. Pathogens and Inoculation Procedures
4.5. Treatment of Plants with Exogenous Hormones
4.6. VIGS of CaSK23 in Pepper Plants
4.7. Transient Expression of CaSK23 in Pepper Leaves
4.8. Construction of Transgenic CaSK23-Overexpressing Tobacco Lines
4.9. Quantitative Real-Time RT-PCR
4.10. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, R.; Zhang, J.; Li, J.; Zhou, G.; Wang, Q.; Bian, W.; Erb, M.; Lou, Y. Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. eLife 2015, 4, e04805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Duran, R.; Macho, A.P.; Boutrot, F.; Segonzac, C.; Somssich, I.E.; Zipfel, C. The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. eLife 2013, 2, e00983. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Baek, K.H.; Park, J.M.; Yi, S.Y.; Yu, S.H.; Kamoun, S.; Choi, D. Capsicum annuum WRKY protein CaWRKY1 is a negative regulator of pathogen defense. New Phytol. 2008, 177, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, Y.; Walker, R.K.; Berkowitz, G.A. Molecular steps in the immune signaling pathway evoked by plant elicitor peptides: Ca2+-dependent protein kinases, nitric oxide, and reactive oxygen species are downstream from the early Ca2+ signal. Plant Physiol. 2013, 163, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Ivashuta, S.; Liu, J.; Lohar, D.P.; Haridas, S.; Bucciarelli, B.; Vanden Bosch, K.A.; Vance, C.P.; Harrison, M.J.; Gantt, J.S. RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell 2005, 17, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Sopory, S.K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 2008, 3, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trewavas, A.J.; Malho, R. Ca2+ signalling in plant cells: The big network! Curr. Opin. Plant Biol. 1998, 1, 428–433. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. Signaling in cells and organisms—Calcium holds the line. Curr. Opin. Plant Biol. 2014, 22, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Poraty-Gavra, L.; Zimmermann, P.; Haigis, S.; Bednarek, P.; Hazak, O.; Stelmakh, O.R.; Sadot, E.; Schulze-Lefert, P.; Gruissem, W.; Yalovsky, S. The Arabidopsis Rho of plants GTPase AtROP6 functions in developmental and pathogen response pathways. Plant Physiol. 2013, 161, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Signaling tip growth in plants. Curr. Opin. Plant Biol. 1998, 1, 525–530. [Google Scholar] [CrossRef]
- Tsukagoshi, H. Control of root growth and development by reactive oxygen species. Curr. Opin. Plant Biol. 2016, 29, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wrzaczek, M.; Brosche, M.; Kangasjarvi, J. ROS signaling loops—Production, perception, regulation. Curr. Opin. Plant Biol. 2013, 16, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Dong, X. Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 2014, 20, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachroo, A.; Robin, G.P. Systemic signaling during plant defense. Curr. Opin. Plant Biol. 2013, 16, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Shah, J. The salicylic acid loop in plant defense. Curr. Opin. Plant Biol. 2003, 6, 365–371. [Google Scholar] [CrossRef]
- Farmer, E.E.; Almeras, E.; Krishnamurthy, V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr. Opin. Plant Biol. 2003, 6, 372–378. [Google Scholar] [CrossRef]
- Reymond, P.; Farmer, E.E. Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1998, 1, 404–411. [Google Scholar] [CrossRef]
- Merchante, C.; Alonso, J.M.; Stepanova, A.N. Ethylene signaling: Simple ligand, complex regulation. Curr. Opin. Plant Biol. 2013, 16, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Giraudat, J. Abscisic acid signaling. Curr. Opin. Cell Biol. 1995, 7, 232–238. [Google Scholar] [CrossRef]
- Belkhadir, Y.; Jaillais, Y. The molecular circuitry of brassinosteroid signaling. New Phytol. 2015, 206, 522–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denance, N.; Sanchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.; Lee, J.; Ung, H.; Chin, K.; Moeder, W.; Yoshioka, K. Crossroads of stress responses, development and flowering regulation—The multiple roles of Cyclic Nucleotide Gated Ion Channel 2. Plant Signal. Behav. 2015, 10, e989758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, Y.; Defraia, C.; Schmelz, E.; Mou, Z. The Arabidopsis MAP kinase kinase 7: A crosstalk point between auxin signaling and defense responses? Plant Signal. Behav. 2008, 3, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Meldau, S.; Ullman-Zeunert, L.; Govind, G.; Bartram, S.; Baldwin, I.T. MAPK-dependent JA and SA signalling in Nicotiana attenuata affects plant growth and fitness during competition with conspecifics. BMC Plant Biol. 2012, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Fiil, B.K.; Petersen, K.; Petersen, M.; Mundy, J. Gene regulation by MAP kinase cascades. Curr. Opin. Plant Biol. 2009, 12, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Bokowiec, M.T.; Han, S.; Zhang, H.; Brannock, J.F.; Chen, X.; Laudeman, T.W.; Timko, M.P. Tobacco transcription factors: Novel insights into transcriptional regulation in the Solanaceae. Plant Physiol. 2008, 147, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Buscaill, P.; Rivas, S. Transcriptional control of plant defence responses. Curr. Opin. Plant Biol. 2014, 20, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.J.; Zhu, Y.X. Transcription factor families in Arabidopsis: Major progress and outstanding issues for future research. Curr. Opin. Plant Biol. 2006, 9, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Yellowlees, D.; Aitken, A.; Donella-Deana, A.; Hemmings, B.A.; Parker, P.J. Separation and characterisation of glycogen synthase kinase 3, glycogen synthase kinase 4 and glycogen synthase kinase 5 from rabbit skeletal muscle. Eur. J. Biochem. 1982, 124, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhao, J.; Peng, P.; Chihara, R.K.; Li, J. BIN2 functions redundantly with other Arabidopsis GSK3-like kinases to regulate brassinosteroid signaling. Plant Physiol. 2009, 150, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.J.; Albert, V.A.; Soltis, P.S.; Soltis, D.E. Phylogenetic diversification of glycogen synthase kinase 3/SHAGGY-like kinase genes in plants. BMC Plant Biol. 2006, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonak, C.; Hirt, H. Glycogen synthase kinase 3/SHAGGY-like kinases in plants: An emerging family with novel functions. Trends Plant Sci. 2002, 7, 457–461. [Google Scholar] [CrossRef]
- Bittner, T.; Campagne, S.; Neuhaus, G.; Rensing, S.A.; Fischer-Iglesias, C. Identification and characterization of two wheat Glycogen Synthase Kinase 3/SHAGGY-like kinases. BMC Plant Biol. 2013, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Bittner, T.; Nadler, S.; Schulze, E.; Fischer-Iglesias, C. Two homolog wheat Glycogen Synthase Kinase 3/SHAGGY—Like kinases are involved in brassinosteroid signaling. BMC Plant Biol. 2015, 15, 247. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Rozhon, W.; Bigeard, J.; Pflieger, D.; Husar, S.; Pitzschke, A.; Teige, M.; Jonak, C.; Hirt, H.; Poppenberger, B. Brassinosteroid-regulated GSK3/Shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. J. Biol. Chem. 2013, 288, 7519–7527. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.H.; Kim, T.W. Functional insights of plant GSK3-like kinases: Multi-taskers in diverse cellular signal transduction pathways. Mol. Plant 2015, 8, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nam, K.H. Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 2002, 295, 1299–1301. [Google Scholar] [PubMed]
- He, J.X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 10185–10190. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, L.; Guo, H.; Yin, Y. MYBL2 is a substrate of GSK3-like kinase BIN2 and acts as a corepressor of BES1 in brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 20142–20147. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, L.; Zhang, Y.; Ouyang, Z.; Hong, Y.; Zhang, H.; Li, D.; Song, F. Tomato SR/CAMTA transcription factors SlSR1 and SlSR3L negatively regulate disease resistance response and SlSR1L positively modulates drought stress tolerance. BMC Plant Biol. 2014, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Kempa, S.; Rozhon, W.; Samaj, J.; Erban, A.; Baluska, F.; Becker, T.; Haselmayer, J.; Schleiff, E.; Kopka, J.; Hirt, H.; et al. A plastid-localized glycogen synthase kinase 3 modulates stress tolerance and carbohydrate metabolism. Plant J. 2007, 49, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Z.; Jiang, M.; Wang, M.; Xue, S.; Zhu, L.L.; Wang, H.Z.; Zou, J.J.; Lee, E.K.; Sack, F.; Le, J. Phosphorylation of Serine 186 of bHLH Transcription Factor SPEECHLESS Promotes Stomatal Development in Arabidopsis. Mol. Plant 2015, 8, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Lee, S.C.; Kim, M.K.; Koh, J.H.; Lee, S.; An, G.; Choe, S.; Kim, S.R. T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol. Biol. 2007, 65, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, J.W.; Ryan, C.A. Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc. Natl. Acad. Sci. USA 1997, 94, 11085–11089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrzaczek, M.; Rozhon, W.; Jonak, C. A Proteasome-regulated glycogen synthase kinase-3 modulates disease response in plants. J. Biol. Chem. 2007, 282, 5249–5255. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.C.; Eini, O.; Rezaian, M.A.; Randles, J.W. A novel shaggy-like kinase interacts with the Tomato leaf curl virus pathogenicity determinant C4 protein. Plant Mol. Biol. 2009, 71, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yang, F.; Li, L.; Chen, H.; Chen, S.; Zhang, J. The Arabidopsis transcription factor BRASSINOSTEROID INSENSITIVE1-ETHYL METHANESULFONATE-SUPPRESSOR1 is a direct substrate of MITOGEN-ACTIVATED PROTEIN KINASE6 and regulates immunity. Plant Physiol. 2015, 167, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.G.; Zhu, T.; Peng, X.J.; Xi, D.H.; Guo, H.; Yin, Y.; Zhang, D.W.; Lin, H.H. Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci. Rep. 2016, 6, 20579. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, D. BRASSINOSTEROID INSENSITIVE2 Interacts with ABSCISIC ACID INSENSITIVE5 to Mediate the Antagonism of Brassinosteroids to Abscisic Acid during Seed Germination in Arabidopsis. Plant Cell 2014, 26, tpc-114. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.; Tang, G.; Barajas-Lopez Jde, D.; et al. GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, J.M.; Ponce, M.R.; Micol, J.L. The ULTRACURVATA2 gene of Arabidopsis encodes an FK506-binding protein involved in auxin and brassinosteroid signaling. Plant Physiol. 2004, 134, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, H.; Wu, D.; Zhang, Z.; Guo, Z.; Yang, N.; Xia, K.; Zhou, X.; Oh, K.; Matsuoka, M.; et al. Zhu, C. Methyl jasmonate inhibits lamina joint inclination by repressing brassinosteroid biosynthesis and signaling in rice. Plant Sci. 2015, 241, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Saidi, Y.; Hearn, T.J.; Coates, J.C. Function and evolution of ‘green’ GSK3/Shaggy-like kinases. Trends Plant Sci. 2012, 17, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.F.; Wang, Y.N.; Yu, L.; Eulgem, T.; Lai, Y.; Liu, Z.Q.; Wang, X.; Qiu, A.L.; Zhang, T.X.; Lin, J.; et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ. 2013, 36, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Jeun, Y.; Hwang, B.K. The pepper patatin-like phospholipase CaPLP1 functions in plant cell death and defense signaling. Plant Mol. Biol. 2014, 84, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Song, G.C.; Yi, H.S.; Ryu, C.M. Field evaluation of the bacterial volatile derivative 3-pentanol in priming for induced resistance in pepper. J. Chem. Ecol. 2014, 40, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, Z.; Yang, S.; Yang, T.; Liang, J.; Wen, J.; Liu, Y.; Li, J.; Shi, L.; Tang, Q.; et al. Pepper CabZIP63 acts as a positive regulator during Ralstonia solanacearum or high temperature-high humidity challenge in a positive feedback loop with CaWRKY40. J. Exp. Bot. 2016, 67, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, S.; Yang, T.; Liang, J.; Cheng, W.; Wen, J.; Liu, Y.; Li, J.; Shi, L.; Tang, Q.; et al. CaCDPK15 positively regulates pepper responses to Ralstonia solanacearum inoculation and forms a positive-feedback loop with CaWRKY40 to amplify defense signaling. Sci. Rep. 2016, 6, 22439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, F.; Liu, Z.; Wang, X.; Eulgem, T.; Lai, Y.; Yu, L.; She, J.; Shi, Y.; Lin, J.; et al. CaWRKY58, encoding a group I WRKY transcription factor of Capsicum annuum, negatively regulates resistance to Ralstonia solanacearum infection. Mol. Plant Pathol. 2013, 14, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.M.; Anand, A.; Kang, L.; Mysore, K.S. Agrodrench: A novel and effective agroinoculation method for virus-induced gene silencing in roots and diverse Solanaceous species. Plant J. 2004, 40, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Hwang, I.S.; Hwang, B.K. Requirement of the cytosolic interaction between PATHOGENESIS-RELATED PROTEIN10 and LEUCINE-RICH REPEAT PROTEIN1 for cell death and defense signaling in pepper. Plant Cell 2012, 24, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Hwang, B.K. Proteomics and functional analyses of pepper abscisic acid-responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell 2011, 23, 823–842. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, B.K. The pepper MLO gene, CaMLO2, is involved in the susceptibility cell-death response and bacterial and oomycete proliferation. Plant J. 2012, 72, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Sarowar, S.; Kim, Y.J.; Kim, E.N.; Kim, K.D.; Hwang, B.K.; Islam, R.; Shin, J.S. Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 2005, 24, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Hwang, B.K. Identification of the pepper SAR8.2 gene as a molecular marker for pathogen infection, abiotic elicitors and environmental stresses in Capsicum annuum. Planta 2003, 216, 387–396. [Google Scholar] [PubMed]
- Lee, S.C.; Hwang, B.K. CASAR82A, a pathogen-induced pepper SAR8.2, exhibits an antifungal activity and its overexpression enhances disease resistance and stress tolerance. Plant Mol. Biol. 2006, 61, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Chi, Y.T.; Kim, D.M.; Choi, S.H.; Lee, J.Y.; Choi, G.W. Ectopic expression of CaRLK1 enhances hypoxia tolerance with increasing alanine production in Nicotiana spp. Plant Mol. Biol. 2014, 86, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Miyasaka, A.; Seo, S.; Ohashi, Y. Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol. 2006, 142, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Pontier, D.; Miao, Z.H.; Lam, E. Trans-dominant suppression of plant TGA factors reveals their negative and positive roles in plant defense responses. Plant J. 2001, 27, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menard, R.; Alban, S.; de Ruffray, P.; Jamois, F.; Franz, G.; Fritig, B.; Yvin, J.C.; Kauffmann, S. Beta-1,3 glucan sulfate, but not beta-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell 2004, 16, 3020–3032. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Kim, Y.H.; Kim, B.R.; Lee, S.Y.; Lim, C.K.; Hur, J.H.; Lee, J.Y. Transgenic tobacco expressing the hrpN(EP) gene from Erwinia pyrifoliae triggers defense responses against botrytis cinerea. Mol. Cells 2007, 24, 232–239. [Google Scholar] [PubMed]
- Dang, F.; Wang, Y.; She, J.; Lei, Y.; Liu, Z.; Eulgem, T.; Lai, Y.; Lin, J.; Yu, L.; Lei, D.; et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiol. Plant 2014, 150, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Traw, M.B.; Chen, J.Q.; Kreitman, M.; Bergelson, J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 2003, 423, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Amornsiripanitch, N.; Dong, X. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006, 2, e123. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- AbuQamar, S.; Chen, X.; Dhawan, R.; Bluhm, B.; Salmeron, J.; Lam, S.; Dietrich, R.A.; Mengiste, T. Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J. 2006, 48, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Sato, M.; Stoddard, T.; Glazebrook, J.; Katagiri, F. Network properties of robust immunity in plants. PLoS Genet. 2009, 5, e1000772. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yang, S.; Yan, Y.; Xiao, Z.; Cheng, J.; Wu, J.; Qiu, A.; Lai, Y.; Mou, S.; Guan, D.; et al. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. J. Exp. Bot. 2015, 66, 3163–3174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, Y.; Zhang, Q.; Ren, S.; Shen, Y.; Qin, L.; Xing, Y. Genome-Wide Analysis of the Expression of WRKY Family Genes in Different Developmental Stages of Wild Strawberry (Fragaria vesca) Fruit. PLoS ONE 2016, 11, e0154312. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gongora, T.; Kim, S.K.; Lozano-Duran, R.; Zipfel, C. Flg22-Triggered Immunity Negatively Regulates Key BR Biosynthetic Genes. Front. Plant Sci. 2015, 6, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.; Chico, J.M.; Solano, R. The jasmonate pathway: The ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 2009, 12, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Munroe, D.J.; Loebbert, R.; Bric, E.; Whitton, T.; Prawitt, D.; Vu, D.; Buckler, A.; Winterpacht, A.; Zabel, B.; Housman, D.E. Systematic screening of an arrayed cDNA library by PCR. Proc. Natl. Acad. Sci. USA 1995, 92, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Park, J.M.; Joung, Y.H.; Lee, S.; Chung, E.; Kim, S.Y.; Yu, S.H.; Choi, D. A plant EPF-type zinc-finger protein, CaPIF1, involved in defence against pathogens. Mol. Plant Pathol. 2005, 6, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kuang, H.; Chen, C.; Yan, J.; Do-Umehara, H.C.; Liu, X.Y.; Dada, L.; Ridge, K.M.; Chandel, N.S.; Liu, J. The kinase Jnk2 promotes stress-induced mitophagy by targeting the small mitochondrial form of the tumor suppressor ARF for degradation. Nat. Immunol. 2015, 16, 458–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, A.; Wu, J.; Lei, Y.; Cai, Y.; Wang, S.; Liu, Z.; Guan, D.; He, S. CaSK23, a Putative GSK3/SHAGGY-Like Kinase of Capsicum annuum, Acts as a Negative Regulator of Pepper’s Response to Ralstonia solanacearum Attack. Int. J. Mol. Sci. 2018, 19, 2698. https://doi.org/10.3390/ijms19092698
Qiu A, Wu J, Lei Y, Cai Y, Wang S, Liu Z, Guan D, He S. CaSK23, a Putative GSK3/SHAGGY-Like Kinase of Capsicum annuum, Acts as a Negative Regulator of Pepper’s Response to Ralstonia solanacearum Attack. International Journal of Molecular Sciences. 2018; 19(9):2698. https://doi.org/10.3390/ijms19092698
Chicago/Turabian StyleQiu, Ailian, Ji Wu, Yufen Lei, Yiting Cai, Song Wang, Zhiqin Liu, Deyi Guan, and Shuilin He. 2018. "CaSK23, a Putative GSK3/SHAGGY-Like Kinase of Capsicum annuum, Acts as a Negative Regulator of Pepper’s Response to Ralstonia solanacearum Attack" International Journal of Molecular Sciences 19, no. 9: 2698. https://doi.org/10.3390/ijms19092698



