In Vitro Studies on Zinc Binding and Buffering by Intestinal Mucins
Abstract
:1. Introduction
2. Results
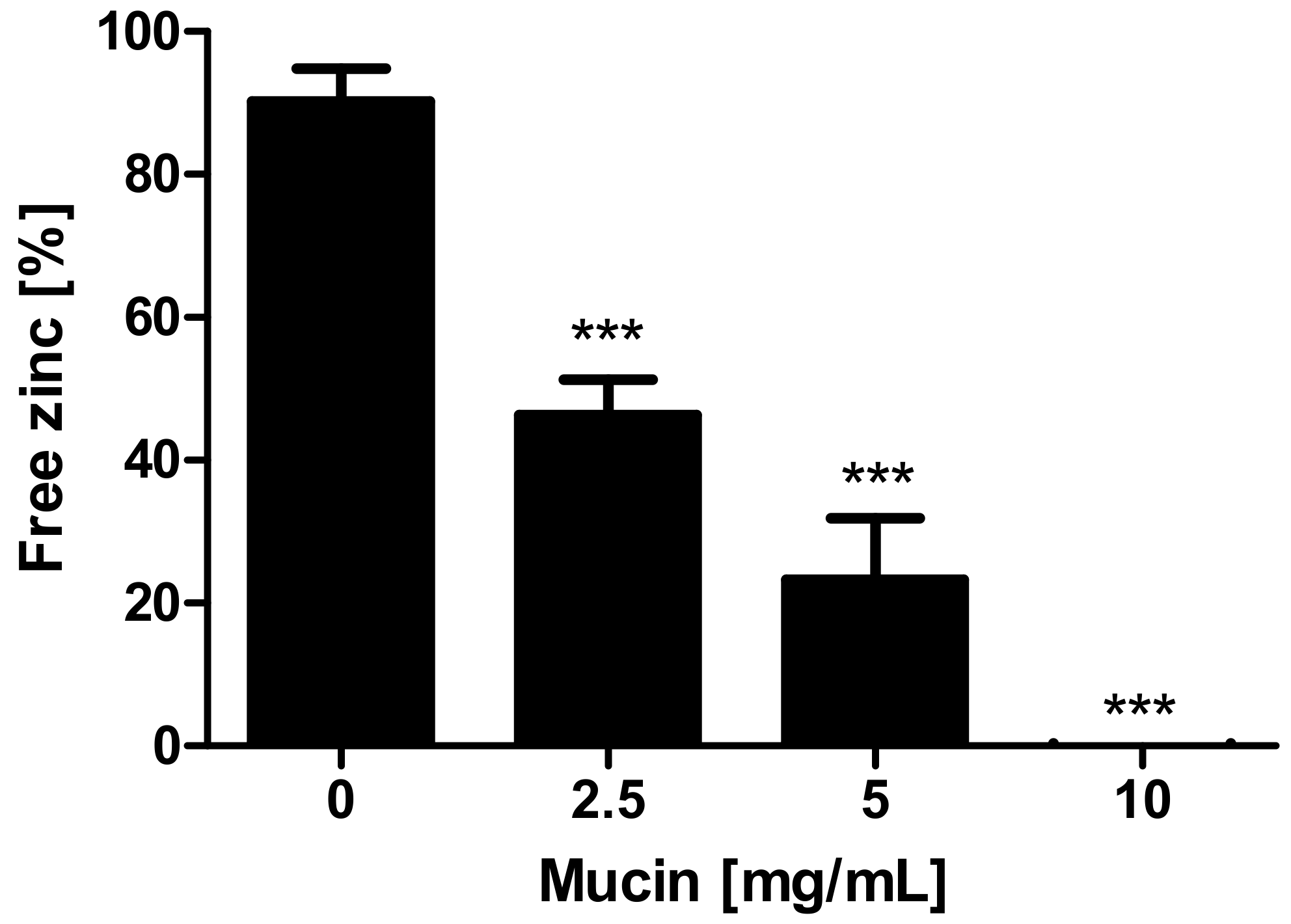
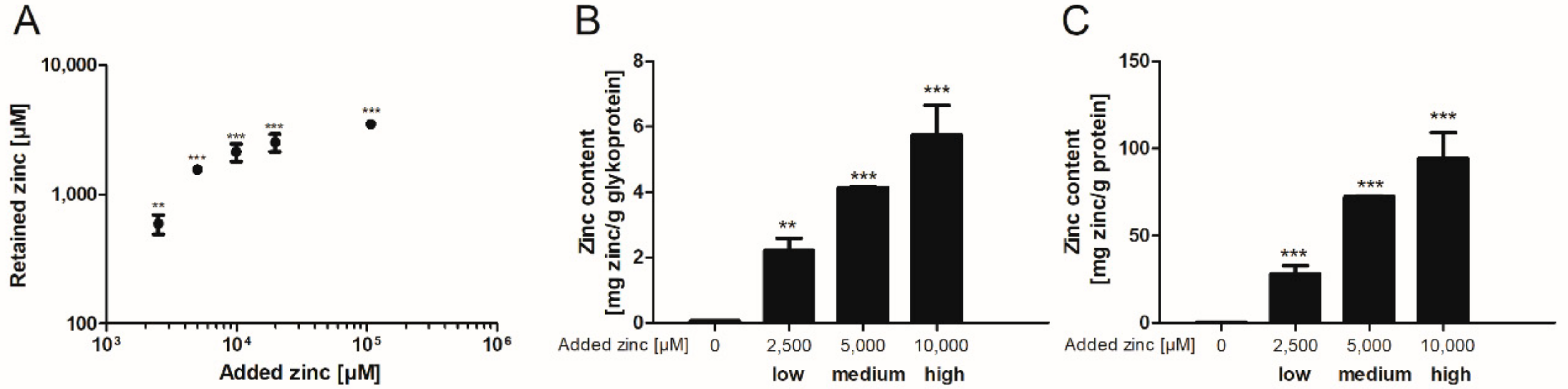
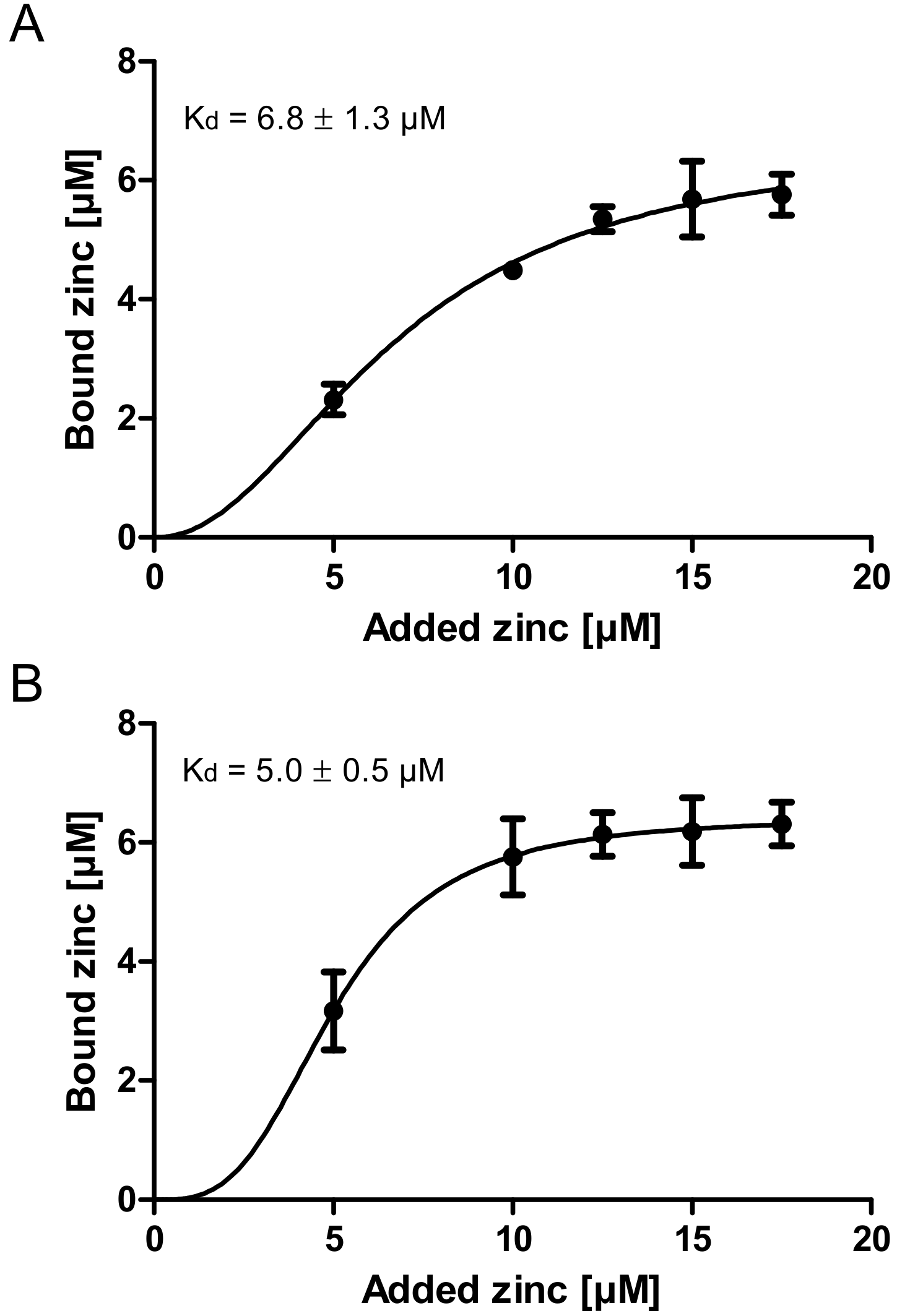
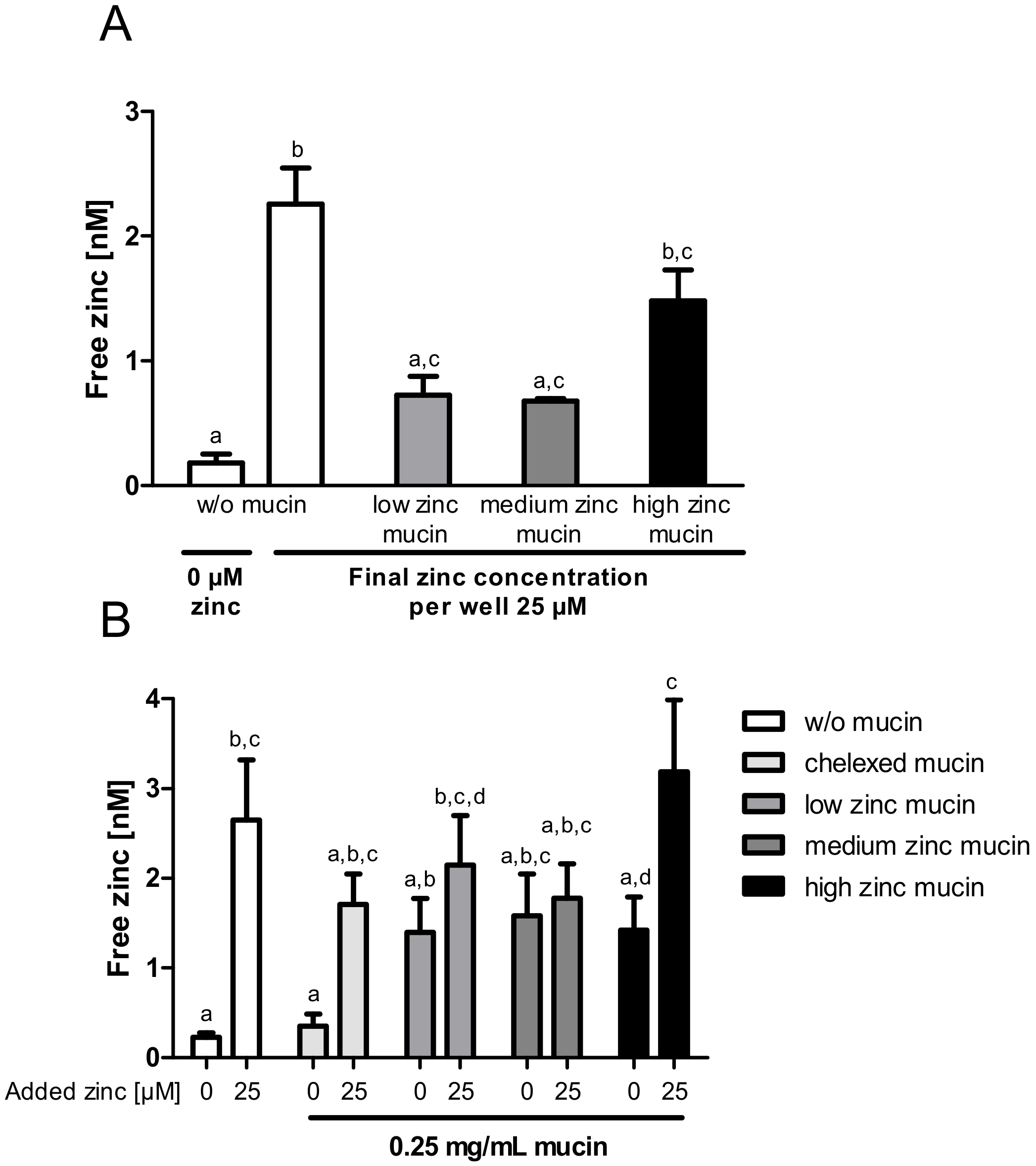
2.1. Zinc Binding by Intestinal Mucins
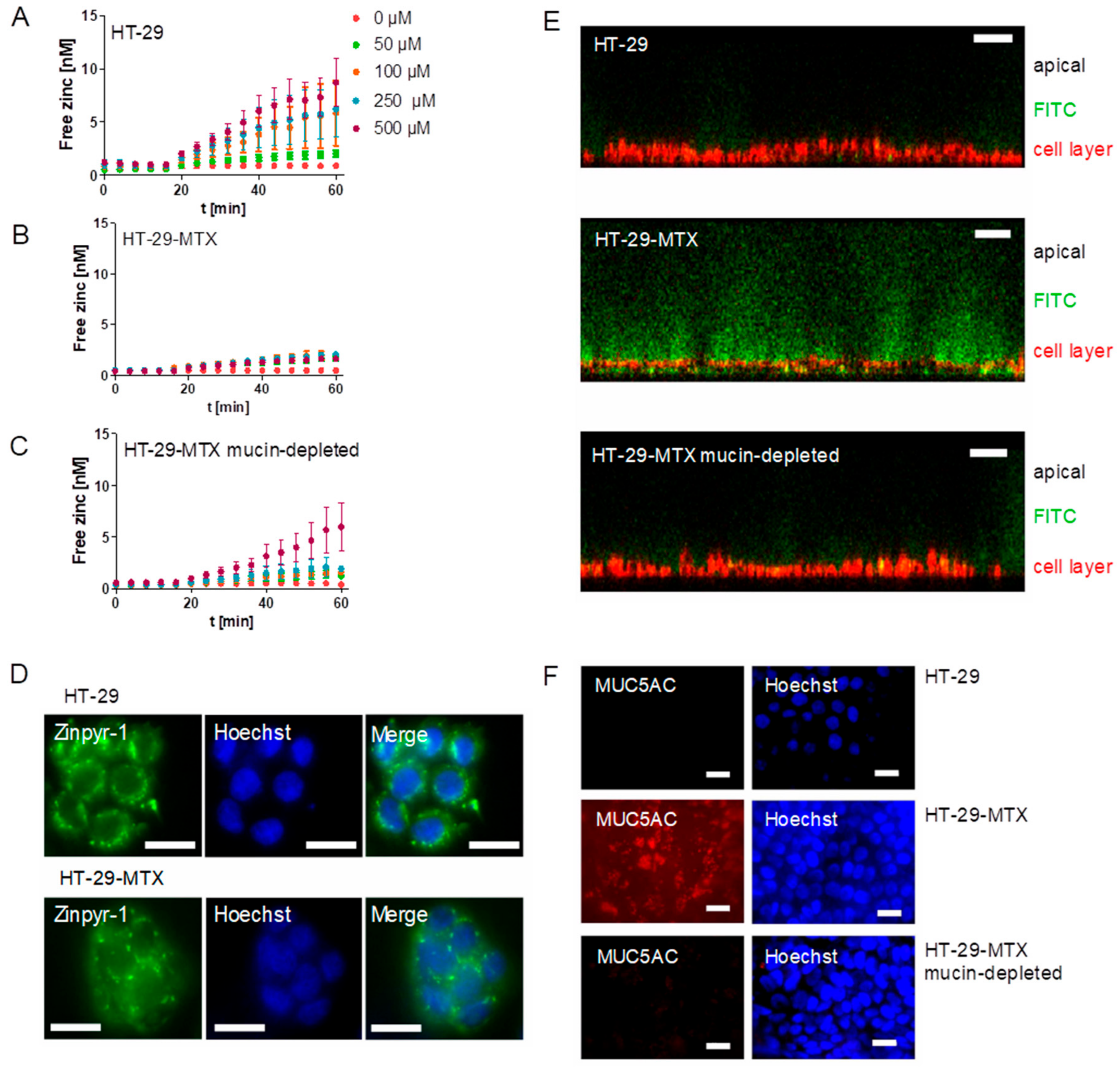
2.2. Role of Zinc Buffering by Mucins on Zinc Uptake into Goblet Cells
2.3. Impact of Extracellular Mucins on Zinc Uptake into Enterocytes
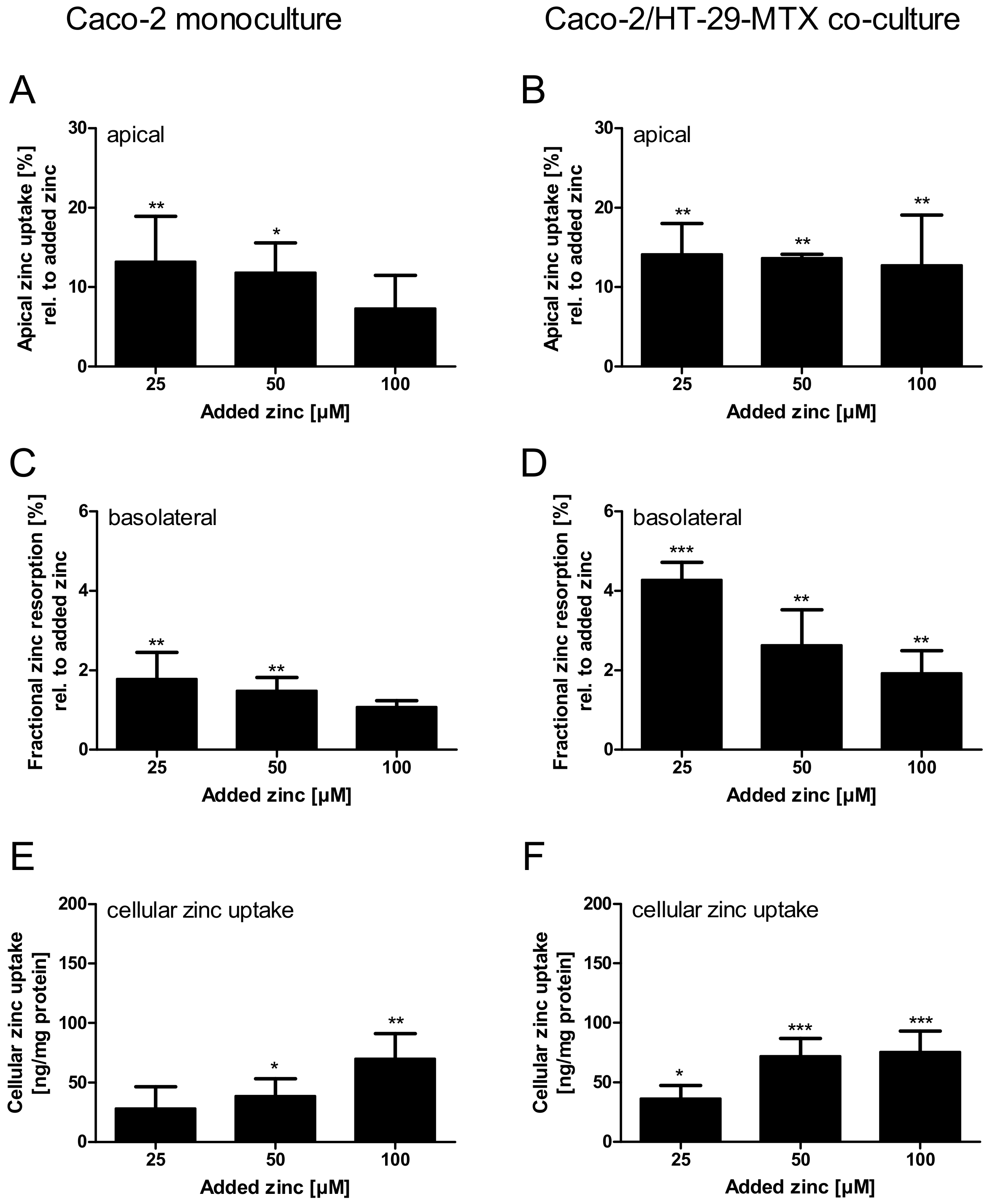
2.4. Comparison of Zinc Resorption in Different Intestinal Cell Culture Models: The Role of Mucins
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. 4-(2-Pyridylazo)Resorcinol (PAR) Assay
4.4. Zinc-Binding Capacity
4.5. Zinc-Binding Affinity of Mucins
4.6. Cellular Zinc Uptake Measured by Zinpyr-1
4.7. Removal of Extracellular Mucins by N-Acetylcysteine
4.8. Immunochemical Staining of the MUC5AC Glycoprotein
4.9. Visualizing Extracellular Mucins with Fluorescein Isothiocyanate (FITC)-Dextran
4.10. Total Cellular Zinc Content Measured by Flame Atomic Absorption Spectrometry (FAAS)
4.11. Zinc Transport Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BCA | bicinchoninic acid |
| BSA | bovine serum albumin |
| CLSM | confocal laser scanning microscopy |
| DMEM | Dulbecco’s modified Eagles medium |
| ECACC | European Collection of Cell Cultures |
| HBSS | Hanks’ balanced salt solution |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| FAAS | flame atomic absorption spectrometry |
| FCS | fetal calf serum |
| FD | (FITC)-Dextran |
| FITC | fluorescein isothiocyanate |
| ICP-MS | inductively-coupled plasma mass spectrometry |
| NAC | N-acetylcysteine |
| NEAA | non-essential amino acids |
| PAR | 4-(2-pyridylazo)resorcinol |
| PAS | periodic acid Schiff |
| Papp | apparent permeability |
| PBS | phosphate buffered saline |
| TBS | tris(hydroxymethyl)aminomethane (Tris)-buffered saline |
| TBST | tris-buffered saline with Tween 20 |
| TEER | transepithelial electrical resistance |
| TPEN | N,N,N′,N′-Tetrakis(2-pyridylmethyl)ethylenediamine |
| ZnT | zinc transporter |
| ZIP | Zrt Irt-like transporter |
| Zincon | 2-carboxy-2′-hydroxy-5′-sulfoformazylbenzene monosodium salt |
References
- Lichten, L.A.; Cousins, R.J. Cousins. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Jugdaohsingh, R.; Thompson, R.P. The regulation of mineral absorption in the gastrointestinal tract. Proc. Nutr. Soc. 1999, 58, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.E.; Umbreit, J.N.; Moore, E.G. A role for mucin in the absorption of inorganic iron and other metal cations. A study in rats. Gastroenterology 1991, 100, 129–136. [Google Scholar] [CrossRef]
- Powell, J.J.; Whitehead, M.W.; Ainley, C.C.; Kendall, M.D.; Nicholson, J.K.; Thompson, R.P.H. Dietary minerals in the gastrointestinal tract: Hydroxypolymerisation of aluminium is regulated by luminal mucins. J. Inorg. Biochem. 1999, 75, 167–180. [Google Scholar] [CrossRef]
- Whitehead, M.W.; Thompson, R.P.; Powell, J.J. Regulation of metal absorption in the gastrointestinal tract. Gut 1996, 39, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Whitehead, M.W.; Lee, S.; Thompson, R.P.H. Mechanisms of gastrointestinal absorption—Dietary minerals and the influence of beverage ingestion. Food Chem. 1994, 51, 381–388. [Google Scholar] [CrossRef]
- Quarterman, J. Metal absorption and the intestinal mucus layer. Digestion 1987, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glover, C.N.; Hogstrand, C. In vivo characterisation of intestinal zinc uptake in freshwater rainbow trout. J. Exp. Biol. 2002, 205, 141–150. [Google Scholar] [PubMed]
- Johansson, M.E.; Sjovall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Madara, J. L. Functional Morphology of Epithelium of the Small Intestine. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Langerholc, T.; Maragkoudakis, P.A.; Wollgast, J.; Gradisnik, L.; Cencic, A. Novel and established intestinal cell line models—An indispensable tool in food science and nutrition. Trends Food Sci. Tech. 2011, 22, S11–S20. [Google Scholar] [CrossRef]
- Sauer, A.K.; Pfaender, S.; Hagmeyer, S.; Tarana, L.; Mattes, A.K.; Briel, F.; Kury, S.; Boeckers, T.M.; Grabrucker, A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hipsc. Biometals 2017, 30, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Duman, A.; Keil, C.; Schwerdtle, T.; Haase, H. The impact of apical and basolateral albumin on intestinal zinc resorption in the Caco-2/HT-29-MTX co-culture model. Metallomics 2018, 10, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. The biochemical effects of extracellular zn(2+) and other metal ions are severely affected by their speciation in cell culture media. Metallomics 2015, 7, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Robineleon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simonassmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human-colon carcinoma cell-line Caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Mahler, G.J.; Shuler, M.L.; Glahn, R.P. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J. Nutr. Biochem. 2009, 20, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, C.; Spahn-Langguth, H.; Regardh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: Permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Walter, E.; Janich, S.; Roessler, B.J.; Hilfiger, J.M.; Amidon, G.L. HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: In vitro−in vivo correlation with permeability data from rats and humans. J. Pharm. Sci. Exp. Pharmacol. 1996, 85, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Lett. Appl. Microbiol. 2009, 49, 695–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calatayud, M.; Vazquez, M.; Devesa, V.; Velez, D. In vitro study of intestinal transport of inorganic and methylated arsenic species by Caco-2/HT29-MTX cocultures. Chem. Res. Toxicol. 2012, 25, 2654–2662. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; Calatayud, M.; Velez, D.; Devesa, V. Intestinal transport of methylmercury and inorganic mercury in various models of Caco-2 and HT29-MTX cells. Toxicology 2013, 311, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Analyzing free zinc(ii) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Welch, R.; Glahn, R. Moving toward a more physiological model: Application of mucin to refine the in vitro digestion/Caco-2 cell culture system. J. Agric. Food Chem. 2006, 54, 8962–8967. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Hansson, G.C. Mucus and the goblet cell. Dig. Dis. 2013, 31, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Holmen Larsson, J.M.; Thomsson, K.A.; Rodriguez-Pineiro, A.M.; Karlsson, H.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. Iii. Gastrointestinal muc5ac and muc2 mucin o-glycan patterns reveal a regiospecific distribution. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G357–G363. [Google Scholar] [CrossRef] [PubMed]
- Kocyla, A.; Pomorski, A.; Krezel, A. Molar absorption coefficients and stability constants of metal complexes of 4-(2-pyridylazo)resorcinol (par): Revisiting common chelating probe for the study of metalloproteins. J. Inorg. Biochem. 2015, 152, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Ju, H.; Lasanajak, Y.; Kudelka, M.R.; Smith, D.F.; Cummings, R.D. Oxidative release of natural glycans for functional glycomics. Nat. Methods 2016, 13, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennebicq-Reig, S.; Lesuffleur, T.; Capon, C.; De Bolos, C.; Kim, I.; Moreau, O.; Richet, C.; Hemon, B.; Recchi, M.A.; Maes, E.; et al. Permanent exposure of mucin-secreting HT-29 cells to benzyl-n-acetyl-alpha-d-galactosaminide induces abnormal O-glycosylation of mucins and inhibits constitutive and stimulated muc5ac secretion. Biochem. J. 1998, 334, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Mantle, M.; Stewart, G.; Zayas, G.; King, M. The disulphide-bond content and rheological properties of intestinal mucins from normal subjects and patients with cystic fibrosis. Biochem. J. 1990, 266, 597–604. [Google Scholar] [PubMed]
- Fogg, F.J.; Hutton, D.A.; Jumel, K.; Pearson, J.P.; Harding, S.E.; Allen, A. Characterization of pig colonic mucins. Biochem. J. 1996, 316, 937–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, R.A.; Christie, G.R.; Phillips, S.R.; Russi, R.M.; Kury, S.; Mathers, J.C.; Taylor, P.M.; Ford, D. A novel zinc-regulated human zinc transporter, hztl1, is localized to the enterocyte apical membrane. J. Biol. Chem. 2002, 277, 22789–22797. [Google Scholar] [CrossRef] [PubMed]
- Casey, C.E.; Walravens, P.A.; Hambidge, K.M.; Silverman, A. The zinc content of human duodenal secretions. Nutr. Res. 1986, 6, 725–728. [Google Scholar] [CrossRef]
- Cousins, R.J. Gastrointestinal factors influencing zinc absorption and homeostasis. Int. J. Vitam. Nutr. Res. 2010, 80, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Navabi, N.; McGuckin, M.A.; Linden, S.K. Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS ONE 2013, 8, e68761. [Google Scholar] [CrossRef] [PubMed]
- Matseshe, J.W.; Phillips, S.F.; Malagelada, J.R.; McCall, J.T. Recovery of dietary iron and zinc from the proximal intestine of healthy man: Studies of different meals and supplements. Am. J. Clin. Nutr. 1980, 33, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Svensson, O.; Arnebrant, T. Mucin layers and multilayers—Physicochemical properties and applications. Curr. Opin. Colloid Interface Sci. 2010, 15, 395–405. [Google Scholar] [CrossRef]
- Sumarokova, M.; Iturri, J.; Weber, A.; Maares, M.; Keil, C.; Luis Toca-Herrera, J. Influencing the adhesion properties and wettability of mucin protein films by variation of the environmental pH. Sci. Rep. 2018, 8, 9660. [Google Scholar] [CrossRef] [PubMed]
- Schomig, V.J.; Kasdorf, B.T.; Scholz, C.; Bidmon, K.; Lieleg, O.; Berensmeier, S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. RSC Adv. 2016, 6, 44932–44943. [Google Scholar] [CrossRef] [Green Version]
- Conrad, M.E.; Umbreit, J.N.; Moore, E.G. Regulation of iron absorption: Proteins involved in duodenal mucosal uptake and transport. J. Am. Coll. Nutr. 1993, 12, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Simovich, M.; Hainsworth, L.N.; Fields, P.A.; Umbreit, J.N.; Conrad, M.E. Localization of the iron transport proteins mobilferrin and dmt-1 in the duodenum: The surprising role of mucin. Am. J. Hematol. 2003, 74, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, V.; Pullakhandam, R.; Nair, K.M. Coordinate expression and localization of iron and zinc transporters explain iron-zinc interactions during uptake in caco-2 cells: Implications for iron uptake at the enterocyte. J. Nutr. Biochem. 2012, 23, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- McCance, R.A.; Widdowson, E.M. The absorption and excretion of zinc. Biochem. J. 1942, 36, 692–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Pelaseyed, T.; Svensson, F.; Johansson, M.E.V. Study of mucin turnover in the small intestine by in vivo labeling. Sci. Rep. 2018, 8, 5760. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS ONE 2012, 7, e41009. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Pieper, R.; Rieger, J.; Vahjen, W.; Davin, R.; Plendl, J.; Meyer, W.; Zentek, J. Effect of dietary zinc oxide on morphological characteristics, mucin composition and gene expression in the colon of weaned piglets. PLoS ONE 2014, 9, e91091. [Google Scholar] [CrossRef] [PubMed]
- Quarterman, J.; Humphries, W.R.; Morrison, J.; Jackson, F.A. The effect of zinc deficiency on intestinal and salivary mucins. Biochem. Soc. Trans. 1973, 1, 101. [Google Scholar] [CrossRef]
- Quarterman, J.; Jackson, F.A.; Morrison, J.N. The effect of zinc deficiency on sheep intestinal mucin. Life Sci. J. 1976, 19, 979–986. [Google Scholar] [CrossRef]
- Sambuy, Y.; de Angelis, M.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model for the intestinal barrier: Influence of cell and culture-related factors on caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Keil, C.; Thomsen, S.; Gunzel, D.; Wiesner, B.; Haase, H. Characterization of Caco-2 cells stably expressing the protein-based zinc probe eCalwy-5 as a model system for investigating intestinal zinc transport. J. Trace Elem. Med. Biol. 2018, 49, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [PubMed]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent sensors for zn2+ based on a fluorescein platform: Synthesis, properties and intracellular distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrens, I.; Pena, A.I.; Alonso, M.J.; Kissel, T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: The effect of mucus on particle adsorption and transport. Pharm. Res. 2002, 19, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, S.; Sheehan, J.K.; Knight, D.; Richardson, P.S.; Thornton, D.J. Heterogeneity of airways mucus: Variations in the amounts and glycoforms of the major oligomeric mucins muc5ac and muc5b. Biochem. J. 2002, 361, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Thornton, D.J.; Carlstedt, I.; Howard, M.; Devine, P.L.; Price, M.R.; Sheehan, J.K. Respiratory mucins: Identification of core proteins and glycoforms. Biochem. J. 1996, 316, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Nollevaux, G.; Deville, C.; El Moualij, B.; Zorzi, W.; Deloyer, P.; Schneider, Y.J.; Peulen, O.; Dandrifosse, G. Development of a serum-free co-culture of human intestinal epithelium cell-lines (Caco-2/HT29-5M21). BMC Cell Biol. 2006, 7, 20. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maares, M.; Keil, C.; Koza, J.; Straubing, S.; Schwerdtle, T.; Haase, H. In Vitro Studies on Zinc Binding and Buffering by Intestinal Mucins. Int. J. Mol. Sci. 2018, 19, 2662. https://doi.org/10.3390/ijms19092662
Maares M, Keil C, Koza J, Straubing S, Schwerdtle T, Haase H. In Vitro Studies on Zinc Binding and Buffering by Intestinal Mucins. International Journal of Molecular Sciences. 2018; 19(9):2662. https://doi.org/10.3390/ijms19092662
Chicago/Turabian StyleMaares, Maria, Claudia Keil, Jenny Koza, Sophia Straubing, Tanja Schwerdtle, and Hajo Haase. 2018. "In Vitro Studies on Zinc Binding and Buffering by Intestinal Mucins" International Journal of Molecular Sciences 19, no. 9: 2662. https://doi.org/10.3390/ijms19092662





