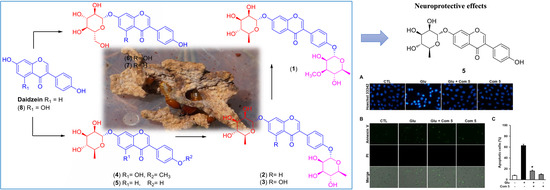
Chemical Identification of Isoflavonoids from a Termite-Associated Streptomyces sp. RB1 and Their Neuroprotective Effects in Murine Hippocampal HT22 Cell Line
Abstract
:1. Introduction
2. Results and Discussion
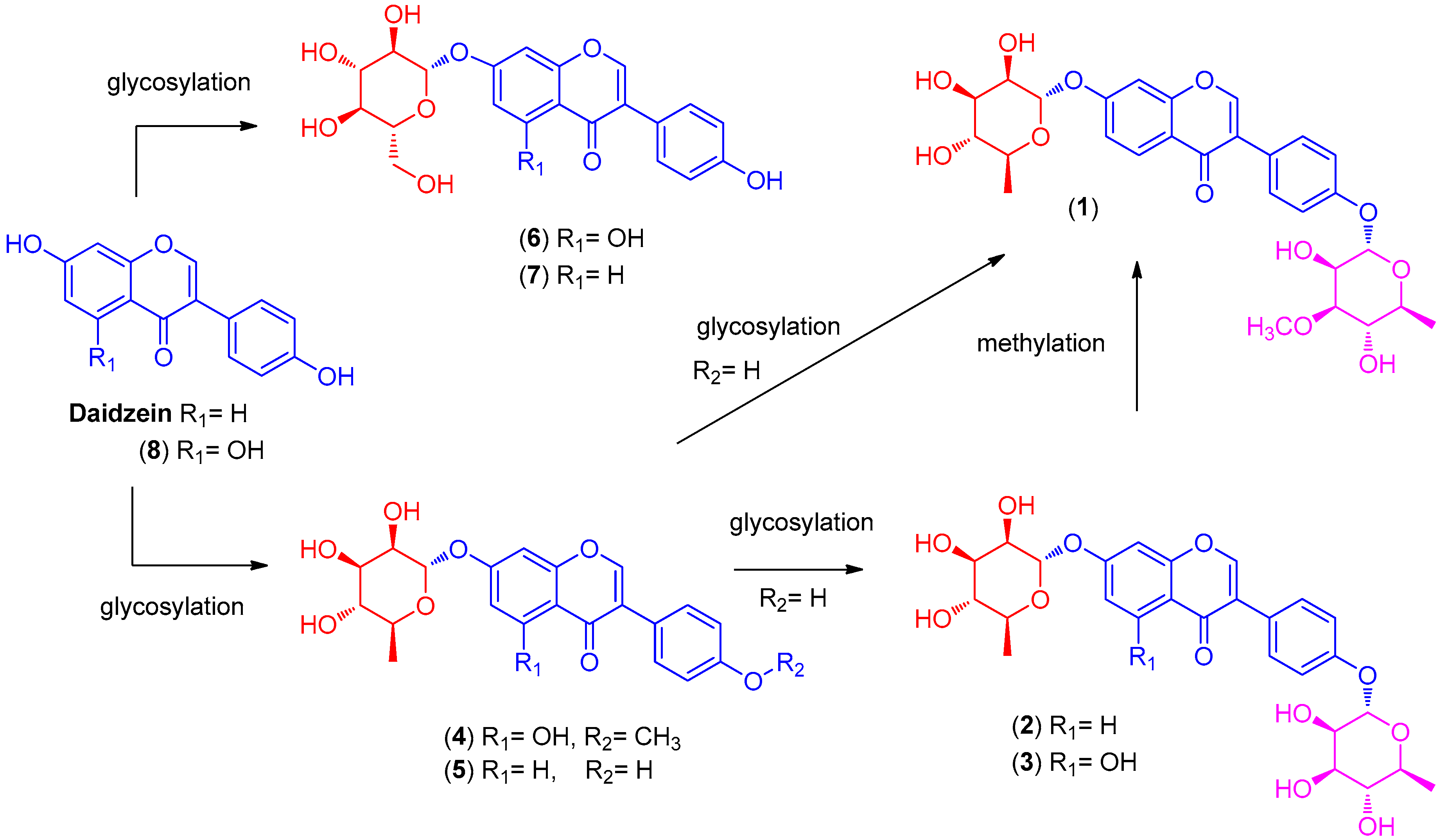
2.1. LC/MS-Guided Isolation of Compounds 1–8
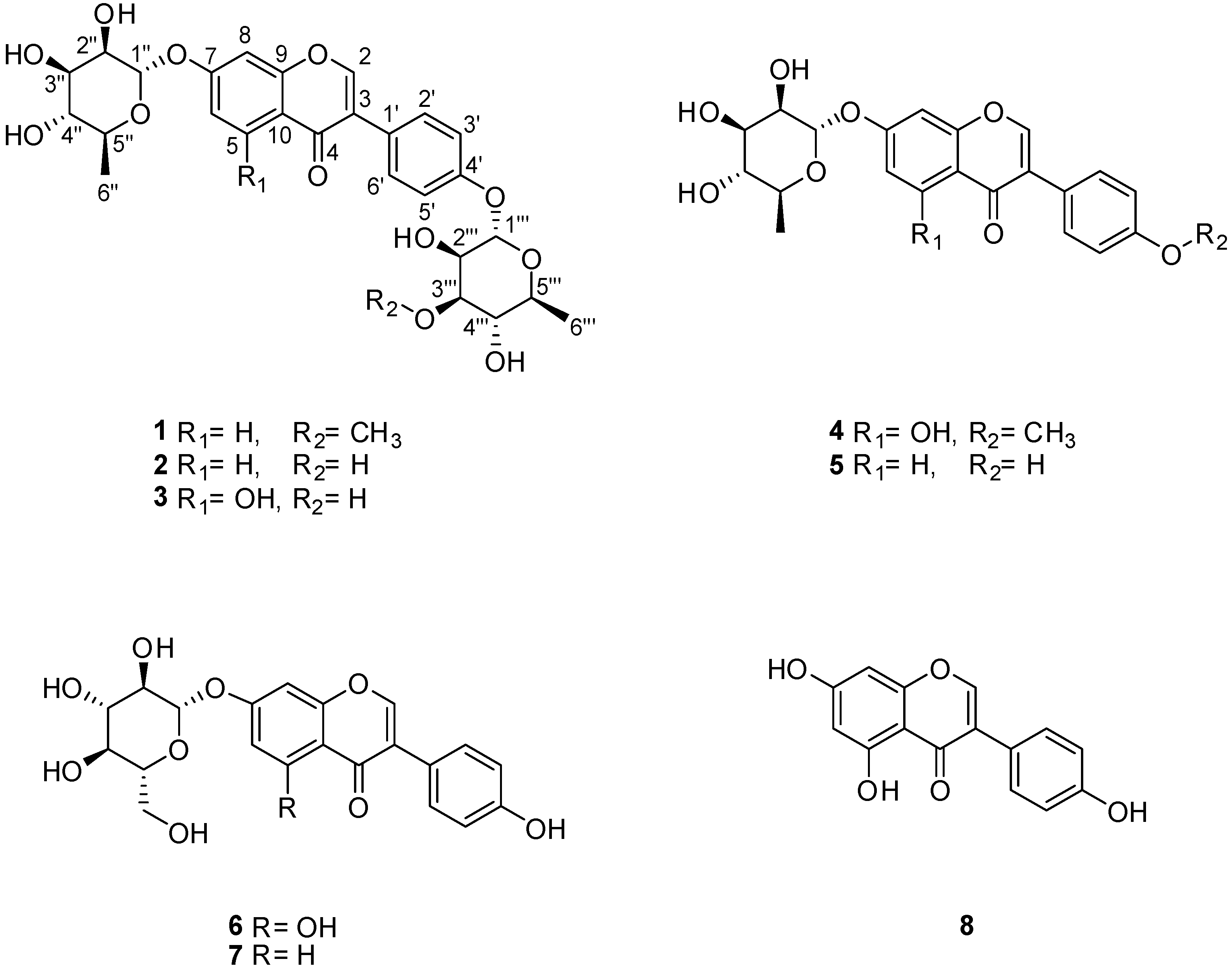
2.2. Structural Elucidation of the Compounds
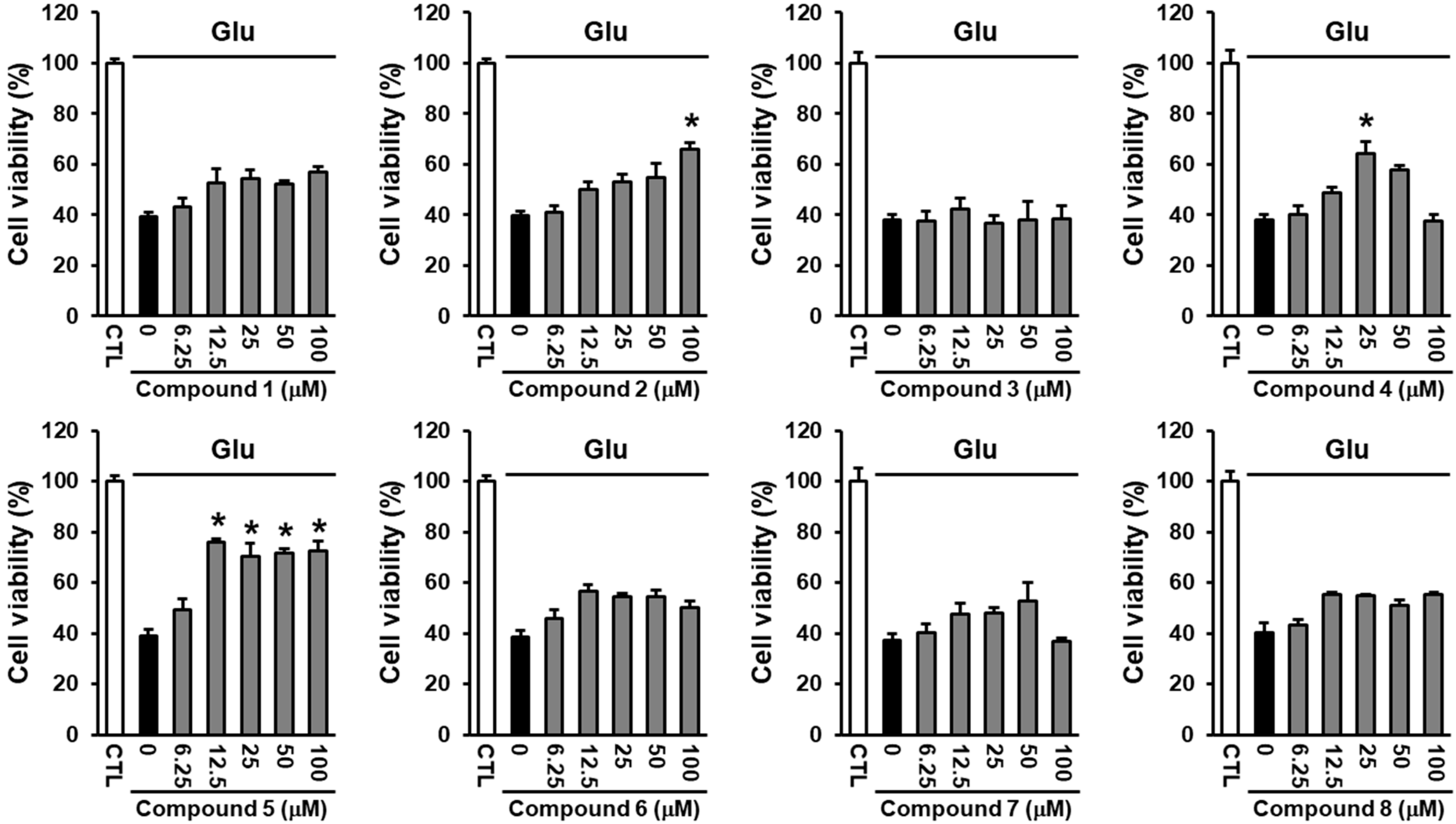
2.3. Neuroprotective Activity of the Compounds Against Glutamate-Induced HT22 Cell Death
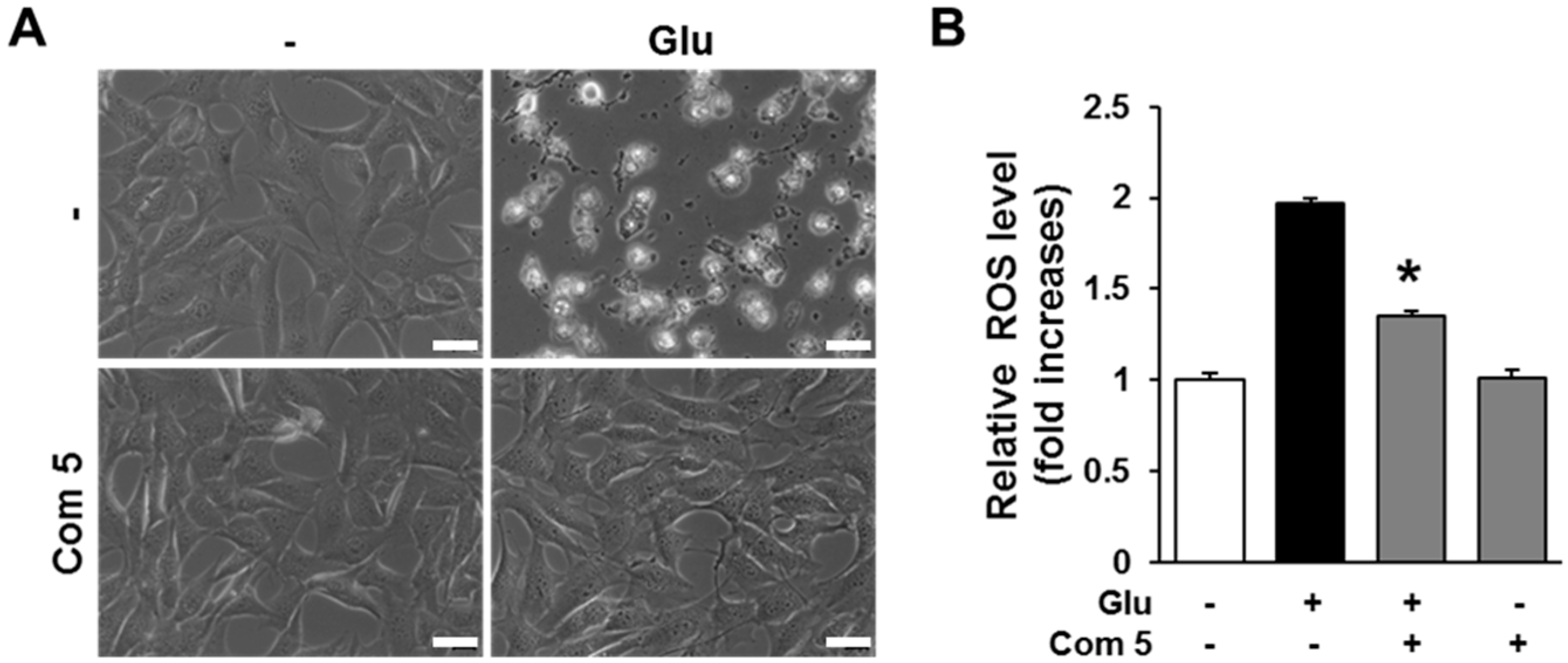
2.4. The Effects of Compound 5 on Glutamate-Induced ROS Accumulation
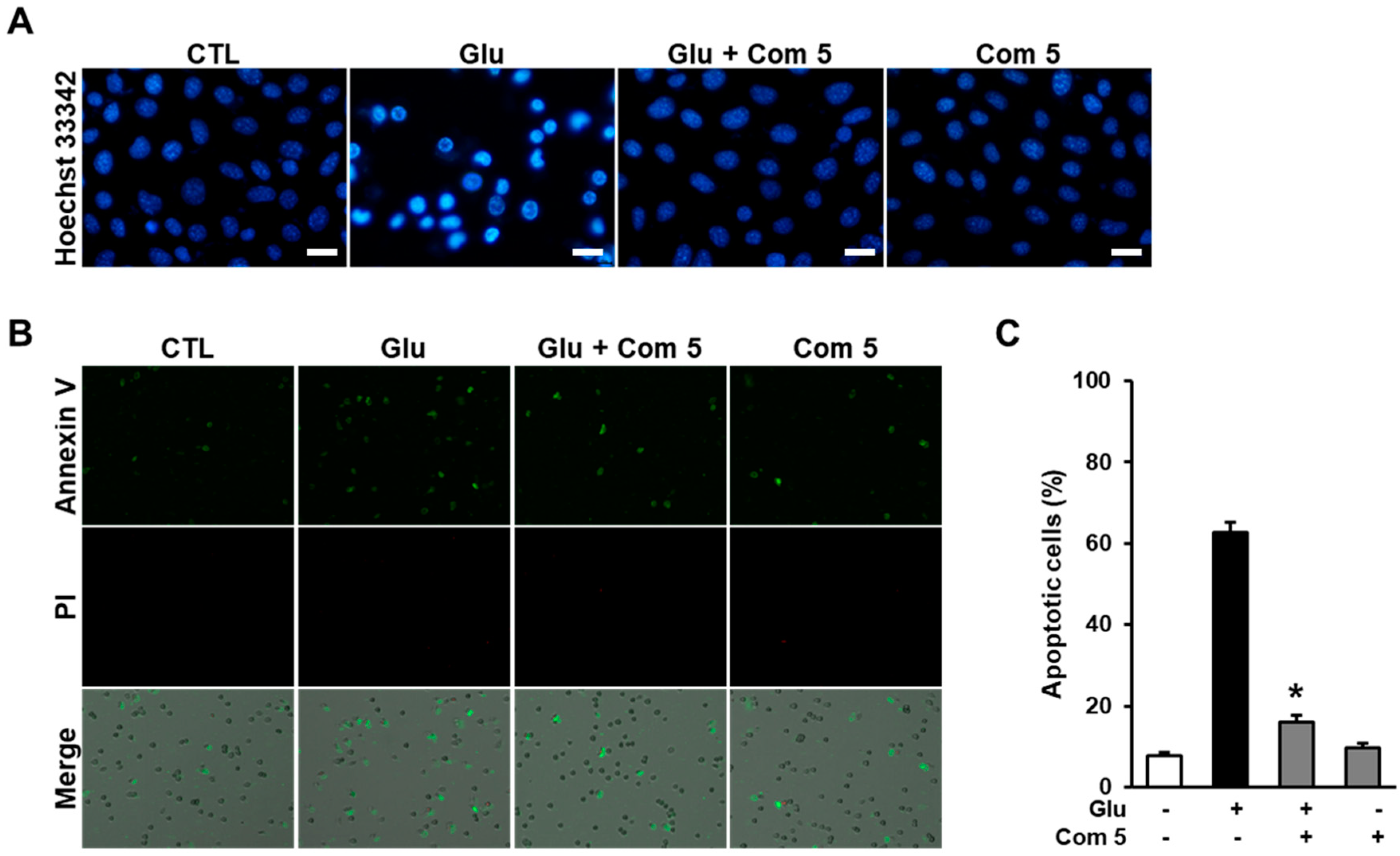
2.5. The Effects of Compound 5 on Glutamate-Induced Damage in HT22 Cells
2.6. Antiviral Activities of the Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Chemical Analysis of Streptomyces sp. RB1
Termisoflavone D (1)
3.3. Acid Hydrolysis of 1 and Sugar Analysis
3.4. Viruses, Cell Lines, and Reagents
3.5. Neuroprotective Activity Assay
3.6. Intracellular ROS Assay
3.7. Number of Apoptotic Cells
3.8. Antiviral Activity Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Becker, M.H.; Harris, R.N. Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PLoS ONE 2006, 5, e10957. [Google Scholar] [CrossRef] [PubMed]
- Wingender, G.; Stepniak, D.; Krebs, P.; Lin, L.; Mcbride, S.; Wei, B.; Braun, J.; Mazmanian, S.K.; Kronenberg, M. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology 2012, 143, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Wyche, T.P.; Ruzzini, A.C.; Schwab, L.; Currie, C.R.; Clardy, J. Tryptorubin A: A polycyclic peptide from a fungus-derived streptomycete. J. Am. Chem. Soc. 2017, 139, 12899–12902. [Google Scholar] [CrossRef] [PubMed]
- Ramadhar, T.R.; Beemelmanns, C.; Currie, C.R.; Clardy, J. Bacterial symbionts in agricultural systems provide a strategic source for antibiotic discovery. J. Antibiot. 2014, 67, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Beemelmanns, C.; Guo, H.; Rischer, M.; Poulsen, M. Natural products from microbes associated with insects. Beilstein J. Org. Chem. 2016, 12, 314–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, J.M.; Clardy, J. Bacterial symbionts and natural products. Chem. Commun. 2011, 47, 7559–7566. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ramadhar, T.R.; Beemelmanns, C.; Cao, S.; Poulsen, M.; Currie, C.R.; Clardy, J. Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem. Sci. 2014, 5, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Beemelmanns, C.; Ramadhar, T.R.; Kim, K.H.; Klassen, J.L.; Cao, S.; Wyche, T.P.; Hou, Y.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; et al. Macrotermycins A–D, glycosylated macrolactams from a termite-associated Amycolatopsis sp. M39. Org. Lett. 2017, 19, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Lee, D.; Benndorf, R.; Jung, W.H.; Beemelmanns, C.; Kang, K.S.; Kim, K.H. Termisoflavones A–C, isoflavonoid glycosides from termite-associated Streptomyces sp. RB1. J. Nat. Prod. 2016, 79, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Um, S.; Fraimout, A.; Sapountzis, P.; Oh, D.C.; Poulsen, M. The fungus-growing termite Macrotermesnatalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci. Rep. 2013, 3, 3250. [Google Scholar] [CrossRef] [PubMed]
- Um, S.; Bach, D.H.; Shin, B.; Ahn, C.H.; Kim, S.H.; Bang, H.S.; Oh, K.B.; Lee, S.K.; Shin, J.; Oh, D.C. Naphthoquinone–oxindole alkaloids, coprisidins A and B, from a gut-associated bacterium in the dung beetle, Copristripartitus. Org. Lett. 2016, 18, 5792–5795. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kang, K.S.; Lee, H.J.; Kim, K.H. Chemical characterization of a renoprotective metabolite from termite-associated Streptomyces sp. RB1 against cisplatin-induced cytotoxicity. Int. J. Mol. Sci. 2018, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; An, N.; Li, Y.; Cheng, S.; Zhang, J.; Zhang, X.; Li, Y. Two new isoflavonoid glucosides from the roots of Achyranthesbidentata and their activities against nitric oxide production. Phytochem. Lett. 2016, 17, 187–189. [Google Scholar] [CrossRef]
- Dai, J.; Shen, D.; Yoshida, W.Y.; Parrish, S.M.; Williams, P.G. Isoflavonoids from Ficusbenjamina and their inhibitory activity on BACE1. Planta Med. 2012, 78, 1357–1362. [Google Scholar] [PubMed]
- Popper, Z.A.; Sadler, I.H.; Fry, S.C. 3-O-Methylrhamnose in lower land plant primary cell walls. Biochem. Syst. Ecol. 2004, 32, 279–289. [Google Scholar] [CrossRef]
- Hazato, T.; Naganawa, H.; Kumagai, M.; Aoyagi, T.; Umezawa, H. β-Galactosidase-inhibiting new isoflavonoids produced by Actinomycetes. J. Antibiot. 1979, 32, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Yoon, T.M.; Kwon, H.J.; Suh, J.W. Talosins A and B: New isoflavonol glycosides with potent antifungal activity from Kitasatosporakifunensis MJM341. J. Antibiot. 2006, 59, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Anandharajan, R.; Pathmanathan, K.; Shankernarayanan, N.P.; Vishwakarma, R.A.; Balakrishnan, A. Upregulation of GLUT-4 and PPARγ by an isoflavone from Pterocarpus marsupium on L6 myotubes: A possible mechanism of action. J. Ethnopharmacol. 2005, 97, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N.; Botting, N.P. An efficient method for the glycosylation of isoflavones. Eur. J. Org. Chem. 2008, 33, 5622–5629. [Google Scholar] [CrossRef]
- Sridhar, C.; Krishnaraju, A.V.; Subbaraju, G.V. Antiinflammatory constituents of Teramnuslabialis. Indian J. Pharm. Sci. 2006, 68, 111–114. [Google Scholar]
- Sattler, R.; Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 2001, 24, 107–129. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, S.; Wang, Y.; Li, X.Q.; Zheng, L.; Zhao, M.G. Neuroprotective effects of formononetin against NMDA-induced apoptosis in cortical neurons. Phytother. Res. 2013, 27, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Hong, H.S.; Kim, D.H.; Kim, H.; Boo, J.H.; Huh, K.; Jung, I.M. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol. Dis. 2004, 16, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, O.; Ballesteros, I.; Cuartero, M.I.; Moraga, A.; Pradillo, J.M.; Ramirez-Franco, J.; Bartolome-Martin, D.; Pascual, D.; Torres, M.; Sanchez-Prieto, J.; et al. Daidzein has neuroprotective effects through ligand-binding-independent PPARγ activation. Neurochem. Int. 2012, 61, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Zhao, T.T.; Park, H.J.; Kim, K.S.; Choi, H.S.; Lee, M.K. Effects of gypenosides on dopaminergic neuronal cell death in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease with long-term l-DOPA treatment. Nat. Prod. Sci. 2016, 22, 187–192. [Google Scholar] [CrossRef]
- Yang, S.J.; Kim, J.; Lee, S.E.; Ahn, J.Y.; Choi, S.Y.; Cho, S.W. Anti-inflammatory and anti-oxidative effects of 3-(naphthalen-2-yl(propoxy)methyl) azetidine hydrochloride on β-amyloid-induced microglial activation. BMB Rep. 2017, 50, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.S.; Kim, D.S.; Ahn, E.H.; Kim, D.W.; Shin, M.J.; Cho, S.B.; Park, J.H.; Lee, C.H.; Yeo, E.J.; Choi, Y.J.; et al. Protective effects of Tat-NQO1 against oxidative stress-induced HT-22 cell damage, and ischemic injury in animals. BMB Rep. 2016, 49, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinwa, P.; Kumar, A. Quercetin along with piperine prevents cognitive dysfunction, oxidative stress and neuro-inflammation associated with mouse model of chronic unpredictable stress. Arch. Pharm. Res. 2017, 40, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wood, M.; Maher, P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J. Neurochem. 1998, 71, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Shahripour, R.B.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Kling, B.; Bucher, D.; Palatzky, P.; Matysik, F.M.; Decker, M.; Wegener, J.; Heilmann, J. Flavonoids, flavonoid metabolites, and phenolic acids inhibit oxidative stress in the neuronal cell line HT-22 monitored by ECIS and MTT assay: A comparative study. J. Nat. Prod. 2014, 77, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Song, J.H.; Choi, J.; Choi, H.J.; Zhu, B.T. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur. J. Pharmacol. 2009, 617, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Argenta, D.F.; Silva, I.T.; Bassani, V.L.; Koester, L.S.; Teixeira, H.F.; Simoes, C.M.O. Antiherpes evaluation of soybean isoflavonoids. Arch. Virol. 2015, 160, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Kim, Y.M.; Kim, J.H.; Kim, J.Y.; Park, J.Y.; Park, S.J.; Ryu, Y.B.; Lee, W.S. Homoisoflavonoids from Caesalpiniasappan displaying viral neuraminidases inhibition. Biol. Pharm. Bull. 2012, 35, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Z.; Du, J.; Hu, Y.; Liu, L.; Yang, F.; Jin, Q. Anti-Japanese-encephalitis-viral effects of kaempferol and daidzin and their RNA-binding characteristics. PLoS ONE 2012, 7, e30259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Y.; Ye, G.; Li, Z.; Zhou, P.; Huang, C. In vivo and in vitro antiviral activities of calycosin-7-O-β-d-glucopyranoside against coxsackie virus B3. Biol. Pharm. Bull. 2009, 32, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yeo, S.G.; Hong, E.H.; Lee, B.R.; Kim, J.W.; Kim, J.; Jeong, H.; Kwon, Y.; Kim, H.; Lee, S.; et al. Antiviral activity of hederasaponin B from hedera helix against enterovirus 71 subgenotypes C3 and C4a. Biomol. Ther. 2014, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattely, E.S.; Fischbachb, M.A.; Walsh, C.T. Total biosynthesis: In vitro reconstitution of polyketide and nonribosomal peptide pathways. Nat. Prod. Rep. 2008, 25, 757–793. [Google Scholar] [CrossRef] [PubMed]
 ) and HMBC (→) correlations for compound 1.
) and HMBC (→) correlations for compound 1.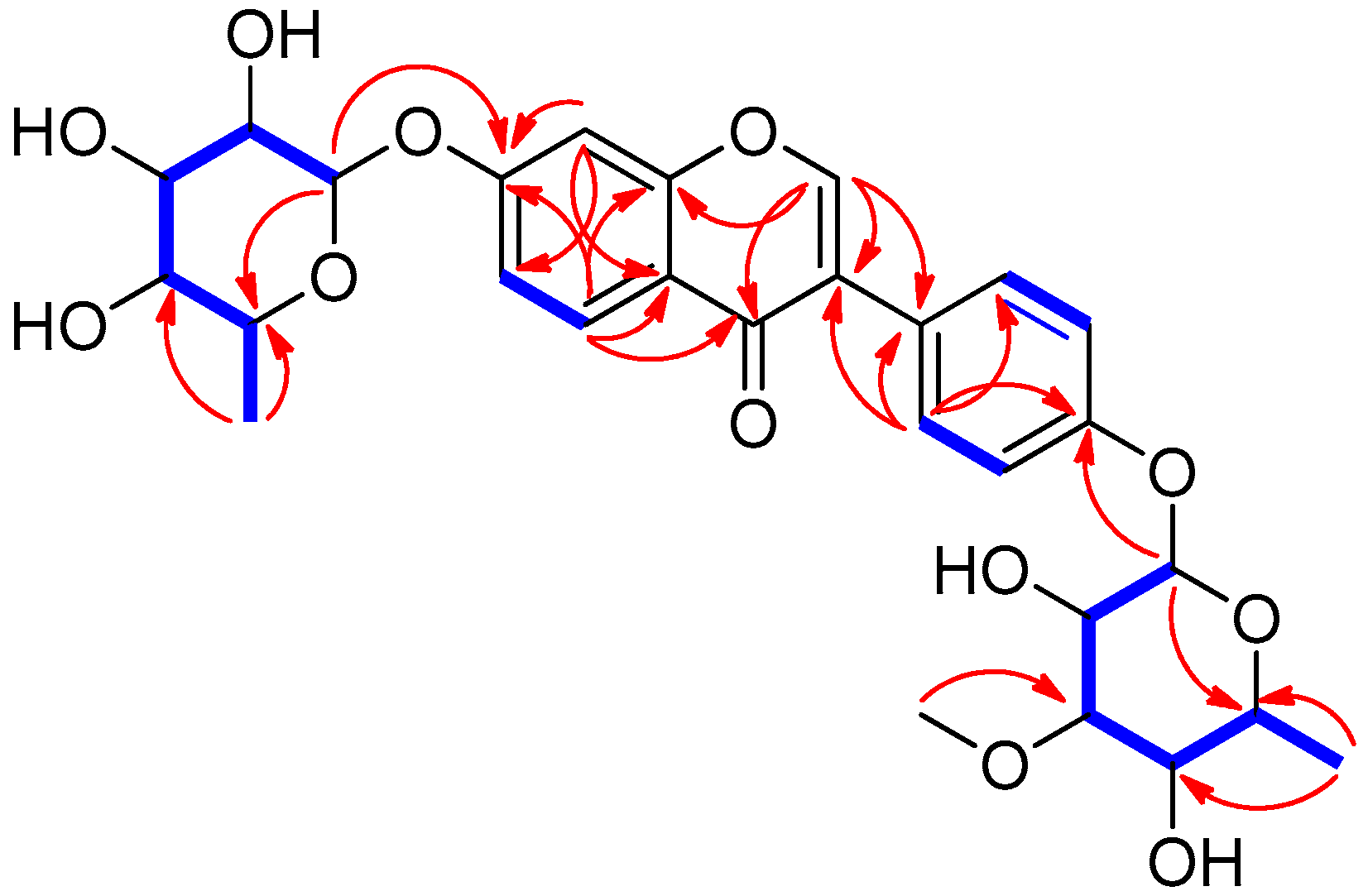
| Position | 1 | |
|---|---|---|
| δCb | δH (J in Hz) | |
| 2 | 155.6 d | 8.17 br s |
| 3 | 126.2 s | |
| 4 | 178.3 s | |
| 5 | 128.8 d | 8.08 d (9.0) |
| 6 | 117.4 d | 7.11 dd (9.0, 2.0) |
| 7 | 162.8 s | |
| 8 | 104.9 d | 7.19 d (2.0) |
| 9 | 159.7 s | |
| 10 | 120.3 s | |
| 1′ | 127.4 s | |
| 2′, 6′ | 131.8 d | 7.42 d (9.0) |
| 3′, 5′ | 117.8 d | 7.07 d (9.0) |
| 4′ | 158.2 s | |
| 1” | 100.3 d | 5.53 br s |
| 2” | 72.0 d | 3.96 m |
| 3” | 72.4 d | 3.76 dd (9.5, 3.0) |
| 4” | 73.8 d | 3.40 t (9.5) |
| 5” | 71.7 d | 3.50 m |
| 6” | 18.4 q | 1.16 d (6.0) |
| 1′′′ | 99.9 d | 5.43 br s |
| 2′′′ | 68.3 d | 4.15 m |
| 3′′′ | 82.2 d | 3.43 m |
| 4′′′ | 72.9 d | 3.42 m |
| 5′′′ | 71.0 d | 3.57 m |
| 6′′′ | 18.4 q | 1.13 d (6.0) |
| 3′′′-OMe | 57.7 q | 3.43 s |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.R.; Song, J.H.; Song, J.-H.; Ko, H.-J.; Baek, J.Y.; Trinh, T.A.; Beemelmanns, C.; Yamabe, N.; Kim, K.H. Chemical Identification of Isoflavonoids from a Termite-Associated Streptomyces sp. RB1 and Their Neuroprotective Effects in Murine Hippocampal HT22 Cell Line. Int. J. Mol. Sci. 2018, 19, 2640. https://doi.org/10.3390/ijms19092640
Lee SR, Song JH, Song J-H, Ko H-J, Baek JY, Trinh TA, Beemelmanns C, Yamabe N, Kim KH. Chemical Identification of Isoflavonoids from a Termite-Associated Streptomyces sp. RB1 and Their Neuroprotective Effects in Murine Hippocampal HT22 Cell Line. International Journal of Molecular Sciences. 2018; 19(9):2640. https://doi.org/10.3390/ijms19092640
Chicago/Turabian StyleLee, Seoung Rak, Ji Hoon Song, Jae-Hyoung Song, Hyun-Jeong Ko, Ji Yun Baek, Tuy An Trinh, Christine Beemelmanns, Noriko Yamabe, and Ki Hyun Kim. 2018. "Chemical Identification of Isoflavonoids from a Termite-Associated Streptomyces sp. RB1 and Their Neuroprotective Effects in Murine Hippocampal HT22 Cell Line" International Journal of Molecular Sciences 19, no. 9: 2640. https://doi.org/10.3390/ijms19092640