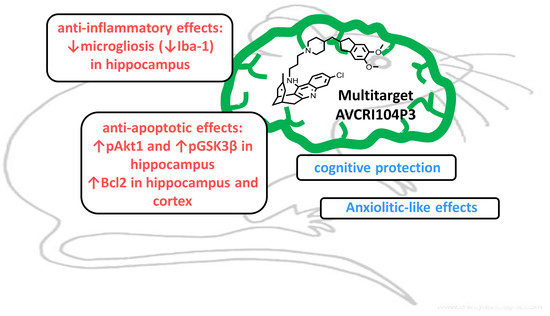
Neuroprotective Effects of the Multitarget Agent AVCRI104P3 in Brain of Middle-Aged Mice
Abstract
:1. Introduction
2. Results
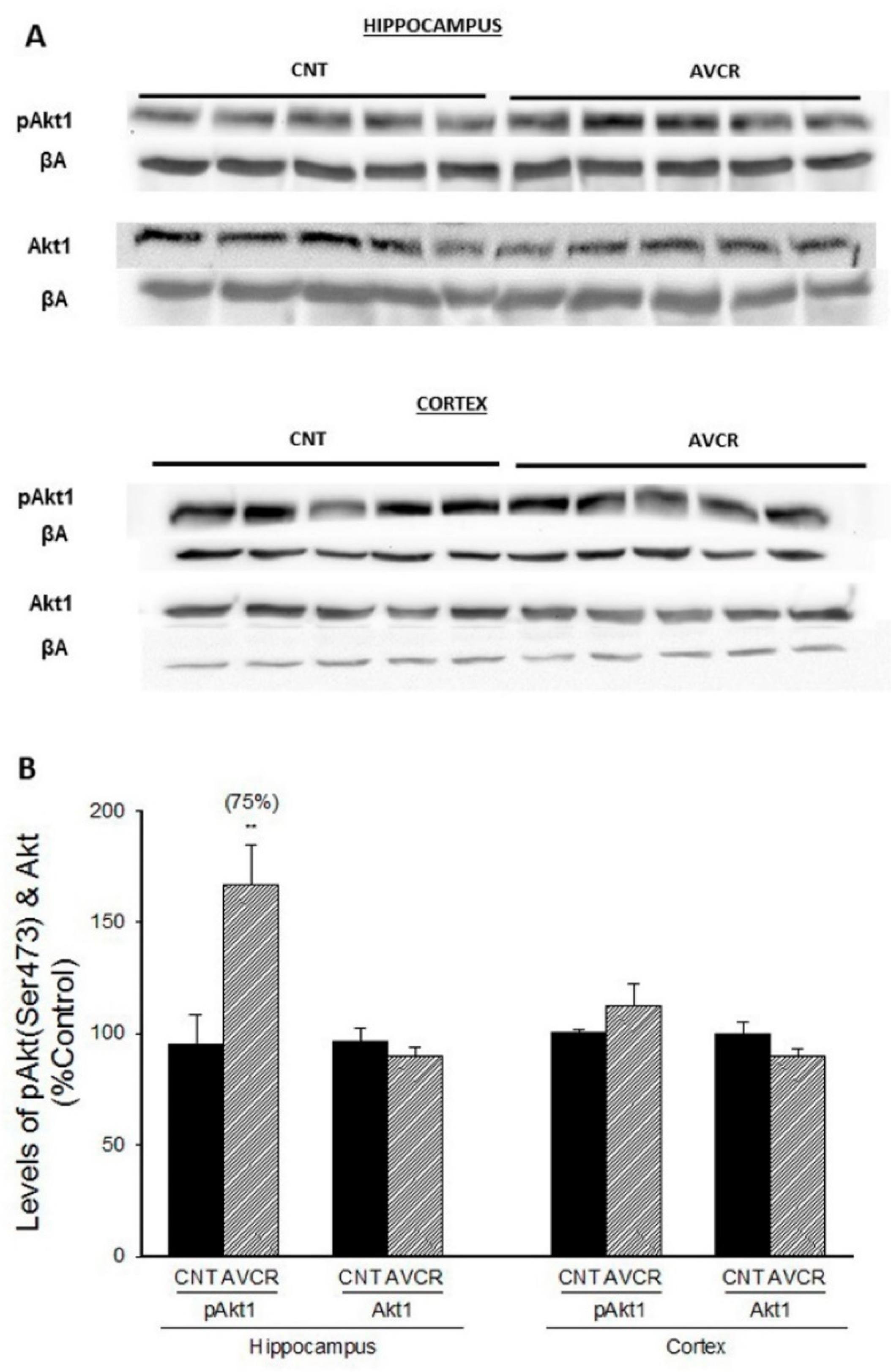
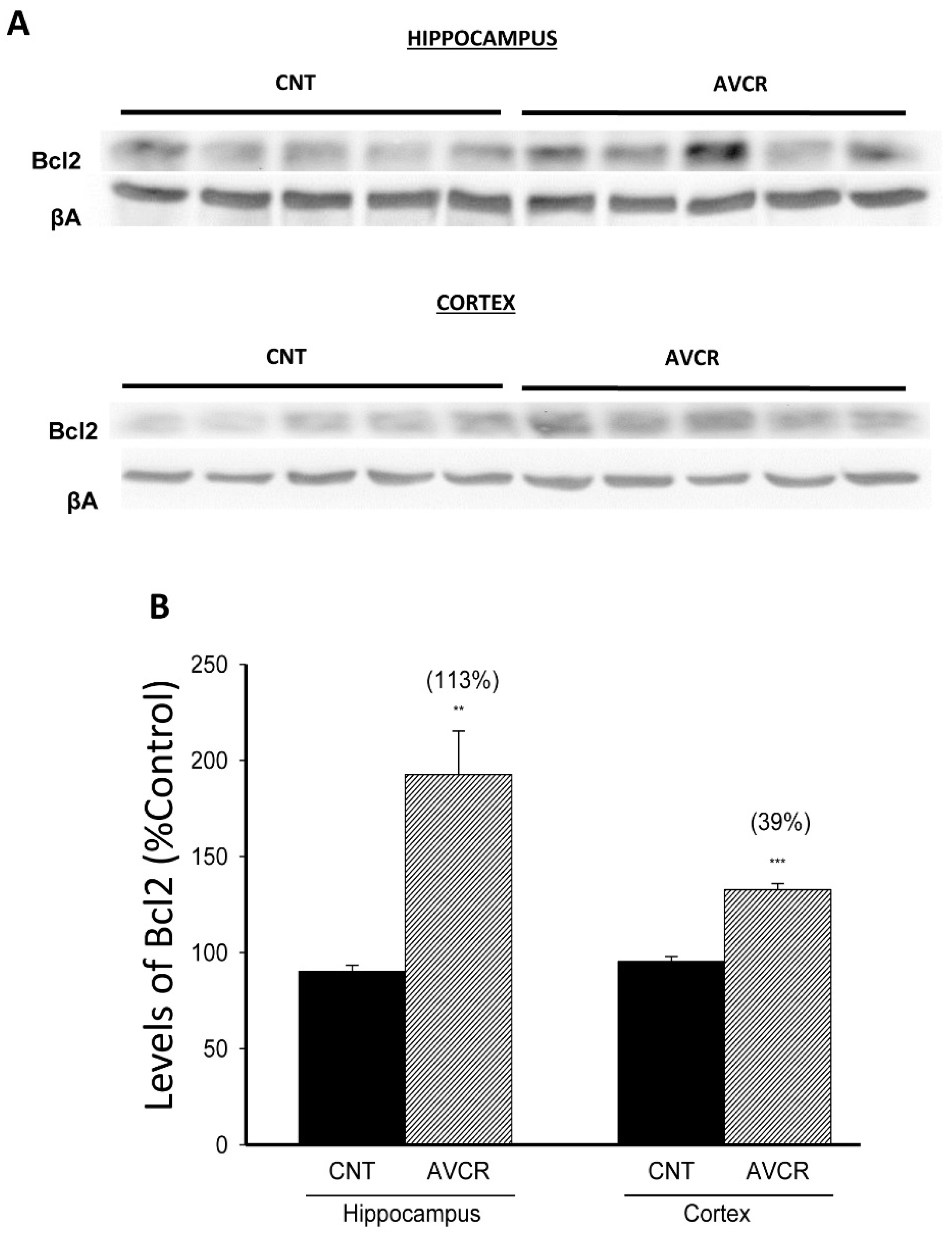
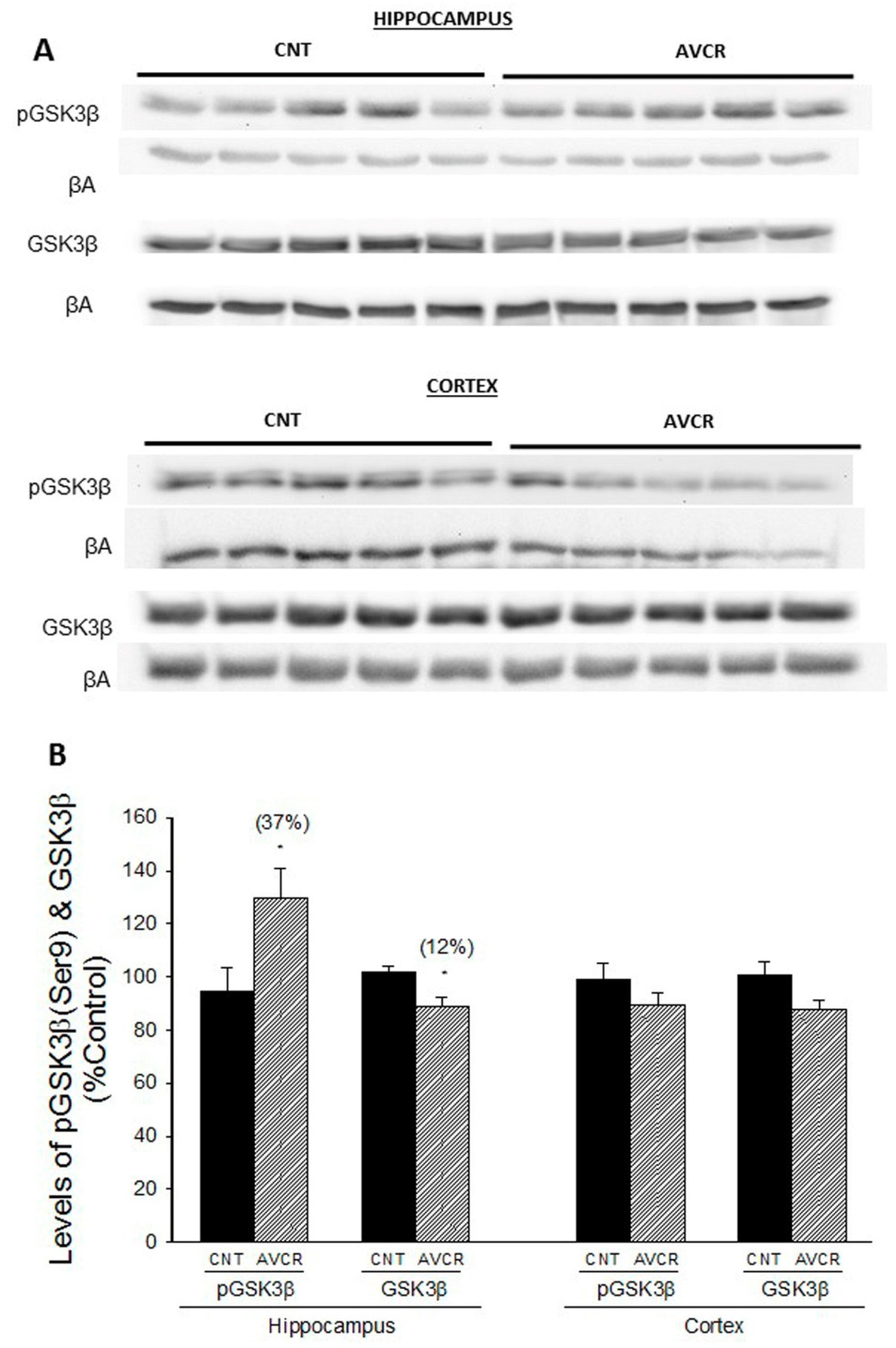
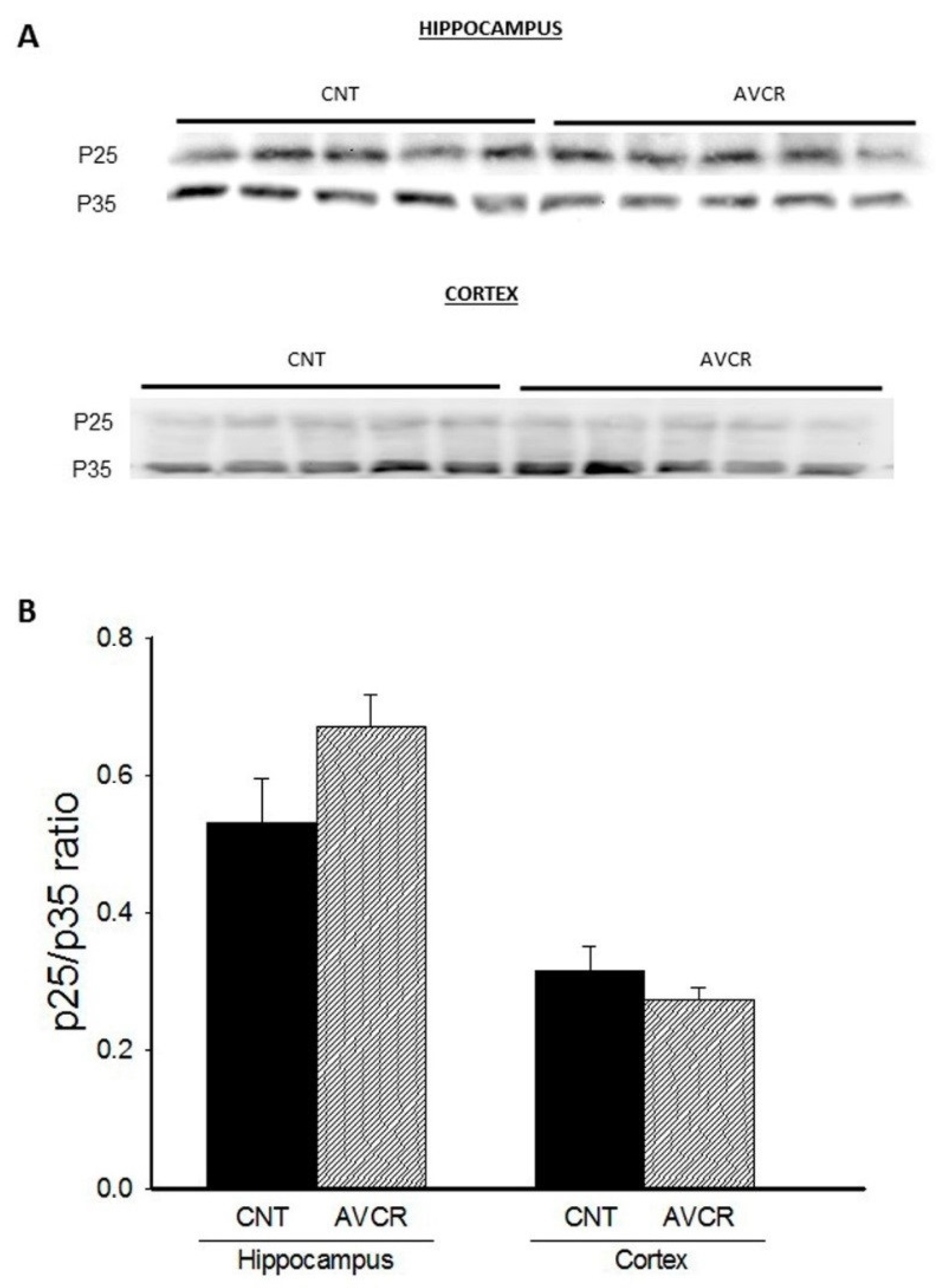
2.1. Effects of AVCRI104P3 on Akt1, Bcl2, GSK3β, Caspases, p25/p35 and Synaptophysin Expression
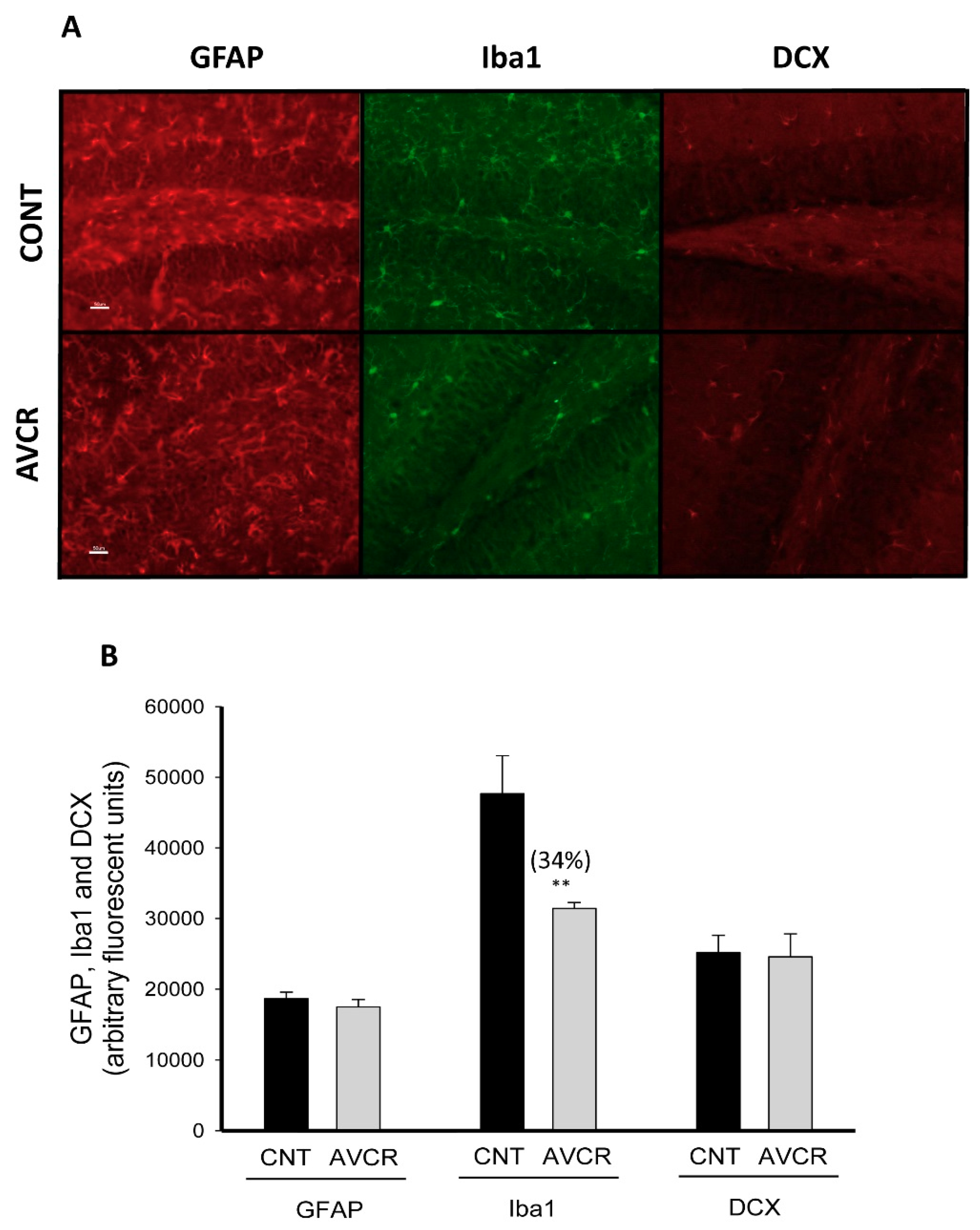
2.2. Effects of AVCRI104P3 on Neuroinflammation (GFAP, Iba-1) and Neurogenesis (DCX) in the Brain
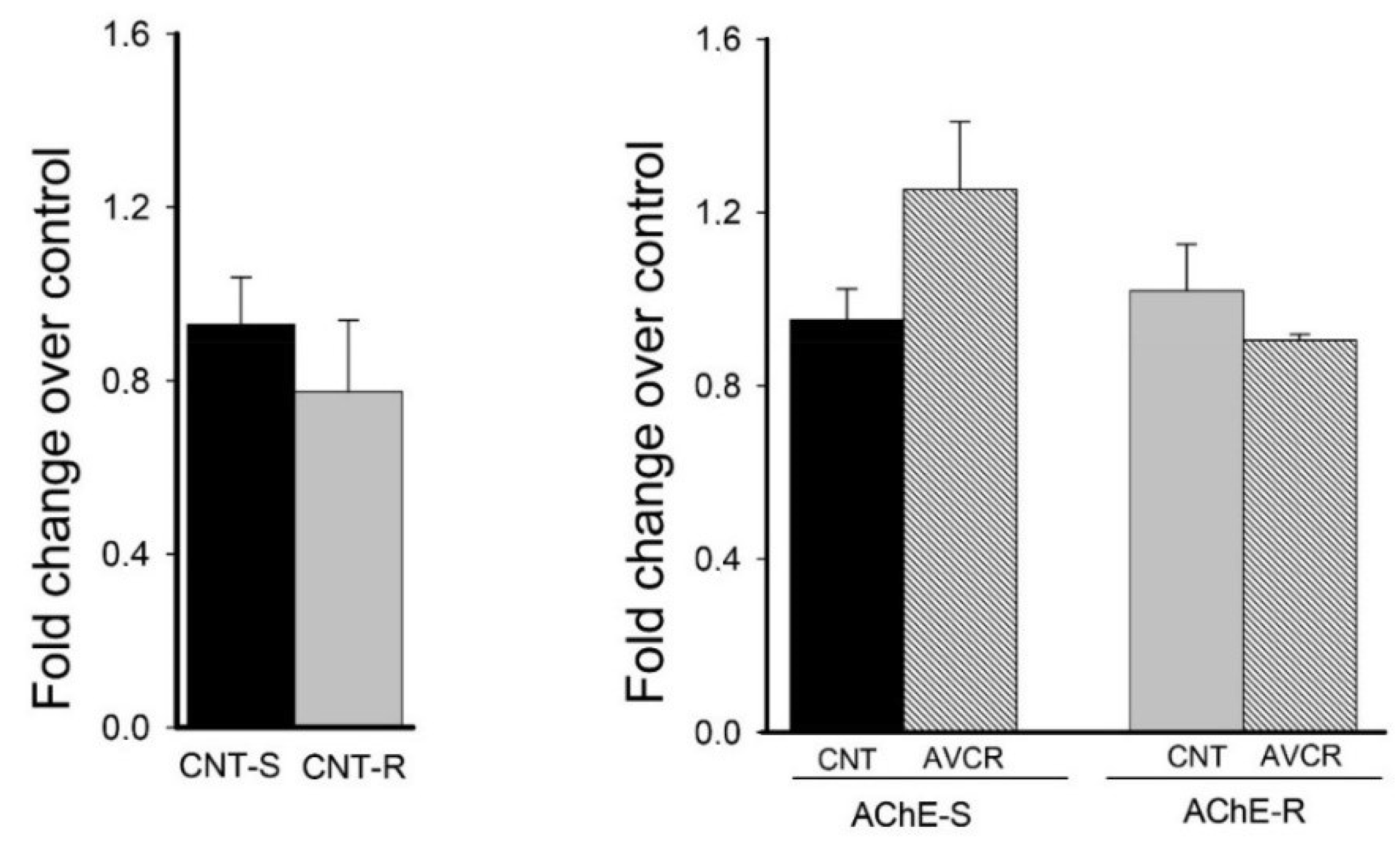
2.3. Effect of AVCRI104P3 on AChE isoforms (AChE-R, AChE-S)
3. Discussion
4. Materials and Methods
4.1. Subjects and Drug Treatment
4.2. Western Blotting (WB)
4.3. Immunohistochemistry
4.4. Caspase Activity
4.5. RT-PCR
4.6. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hedden, T.; Gabrieli, J.D.E. Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Williams, J.W., Jr.; Burke, J.R.; Holsinger, T.; Benjamin, S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann. Intern. Med. 2010, 153, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Froelich, L.; Burns, A. Pharmacological treatment of dementia. Curr. Opin. Psychiatry 2012, 25, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.Y.; Fu, W.M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Lao, K.; Ji, N.; Zhang, X.; Qiao, W.; Tang, Z.; Gou, X. Drug development for Alzheimer’s disease: Review. J. Drug Target. 2018, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; De Bartolo, P.; Caporali, P.; Tartaglione, A.M.; Oddi, D.; D’Amato, F.R.; Nobili, A.; D’Amelio, M.; Petrosini, L. Neuroprotective effects of donepezil against cholinergic depletion. Alzheimers Res. Ther. 2013, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torrero, D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 2008, 15, 2433–2455. [Google Scholar] [CrossRef] [PubMed]
- Odorcyk, F.K.; Nicola, F.; Duran-Carabali, L.E.; Figueiró, F.; Kolling, J.; Vizuete, A.; Konrath, E.L.; Gonçalves, C.A.; Wyse, A.T.S.; Netto, C.A. Galantamine administration reduces reactive astrogliosis and upregulates the anti-oxidant enzyme catalase in rats submitted to neonatal hypoxia ischemia. Int. J. Dev. Neurosci. 2017, 62, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G.; Greig, N.H.; Guillozet-Bongaarts, A.L.; Enz, A.; Darvesh, S. Cholinesterases: Roles in the brain during health and disease. Curr. Alzheimer Res. 2005, 2, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Camps, P.; Cusack, B.; Mallender, W.D.; El Achab, R.E.; Morral, J.; Muñoz-Torrero, D.; Rosenberry, T.L. Huprine X is a novel high-affinity inhibitor of acetylcholinesterase that is of interest for treatment of Alzheimer’s disease. Mol. Pharmacol. 2000, 57, 409–417. [Google Scholar]
- Ratia, M.; Giménez-Llort, L.; Camps, P.; Muñoz-Torrero, D.; Clos, M.V.; Badia, A. Behavioural effects and regulation of PKCα and MAPK by huprine X in middle aged mice. Pharmacol. Biochem. Behav. 2010, 95, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ratia, M.; Giménez-Llort, L.; Camps, P.; Muñoz-Torrero, D.; Pérez, B.; Clos, M.V.; Badia, A. Huprine X and huperzine A improve cognition and regulate some neurochemical processes related with Alzheimer’s disease in triple transgenic mice (3xTg-AD). Neurodegener. Dis. 2013, 11, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, M.M.; Clos, M.V.; Ratia, M.; Gonzalez, D.; Lithner, C.U.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Giménez-Llort, L.; Nordberg, A. Effect of huprine X on β-amyloid, synaptophysin and α7 neuronal nicotinic acetylcholine receptors in the brain of 3xTg-AD and APPswe transgenic mice. Neurodegener. Dis. 2010, 7, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Relat, J.; Pérez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Clos, M.V. Huprine X attenuates the neurotoxicity induced by kainic acid, especially brain inflammation. Basic Clin. Pharmacol. Toxicol. 2018, 122, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Viayna, E.; Sola, I.; Bartolini, M.; De Simone, A.; Tapia-Rojas, C.; Serrano, F.G.; Sabaté, R.; Juárez-Jiménez, J.; Pérez, B.; Luque, F.J.; et al. Synthesis and multi-target biological profiling of a novel family of rhein derivatives as disease-modifying anti-Alzheimer agents. J. Med. Chem. 2014, 57, 2549–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola, I.; Aso, E.; Frattini, D.; López-González, I.; Espargaró, A.; Sabaté, R.; Di Pietro, O.; Luque, F.J.; Clos, M.V.; Ferrer, I.; et al. Novel levetiracetam derivatives that are effective against the Alzheimer-like phenotype in mice: Synthesis, in vitro, ex vivo and in vivo efficacy studies. J. Med. Chem. 2015, 58, 6018–6032. [Google Scholar] [CrossRef] [PubMed]
- Serrano, F.G.; Tapia-Rojas, C.; Carvajal, F.J.; Cisternas, P.; Viayna, E.; Sola, I.; Muñoz-Torrero, D.; Inestrosa, N.C. Rhein-huprine derivatives reduce cognitive impairment, synaptic failure and amyloid pathology in AβPPswe/PS-1 mice of different ages. Curr. Alzheimer Res. 2016, 13, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torrero, D. Multitarget anti-Alzheimer hybrid compounds: Do they work in vivo? In Design of Hybrid Molecules for Drug Development; Decker, M., Ed.; Elsevier: Amsterdam, The Netherland, 2017; Chapter 6; pp. 165–190. [Google Scholar]
- Viayna, E.; Gómez, T.; Galdeano, C.; Ramírez, L.; Ratia, M.; Badia, A.; Clos, M.V.; Verdaguer, E.; Junyent, F.; Camins, A.; et al. Novel huprine derivatives with inhibitory activity toward β-amyloid aggregation and formation as disease-modifying anti-Alzheimer drug candidates. ChemMedChem 2010, 5, 1855–1870. [Google Scholar] [CrossRef] [PubMed]
- Sola, I.; Viayna, E.; Gómez, T.; Galdeano, C.; Cassina, M.; Camps, P.; Romeo, M.; Diomede, L.; Salmona, M.; Franco, P.; et al. Multigram synthesis and in vivo efficacy studies of a novel multitarget anti-Alzheimer’s compound. Molecules 2015, 20, 4492–4515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
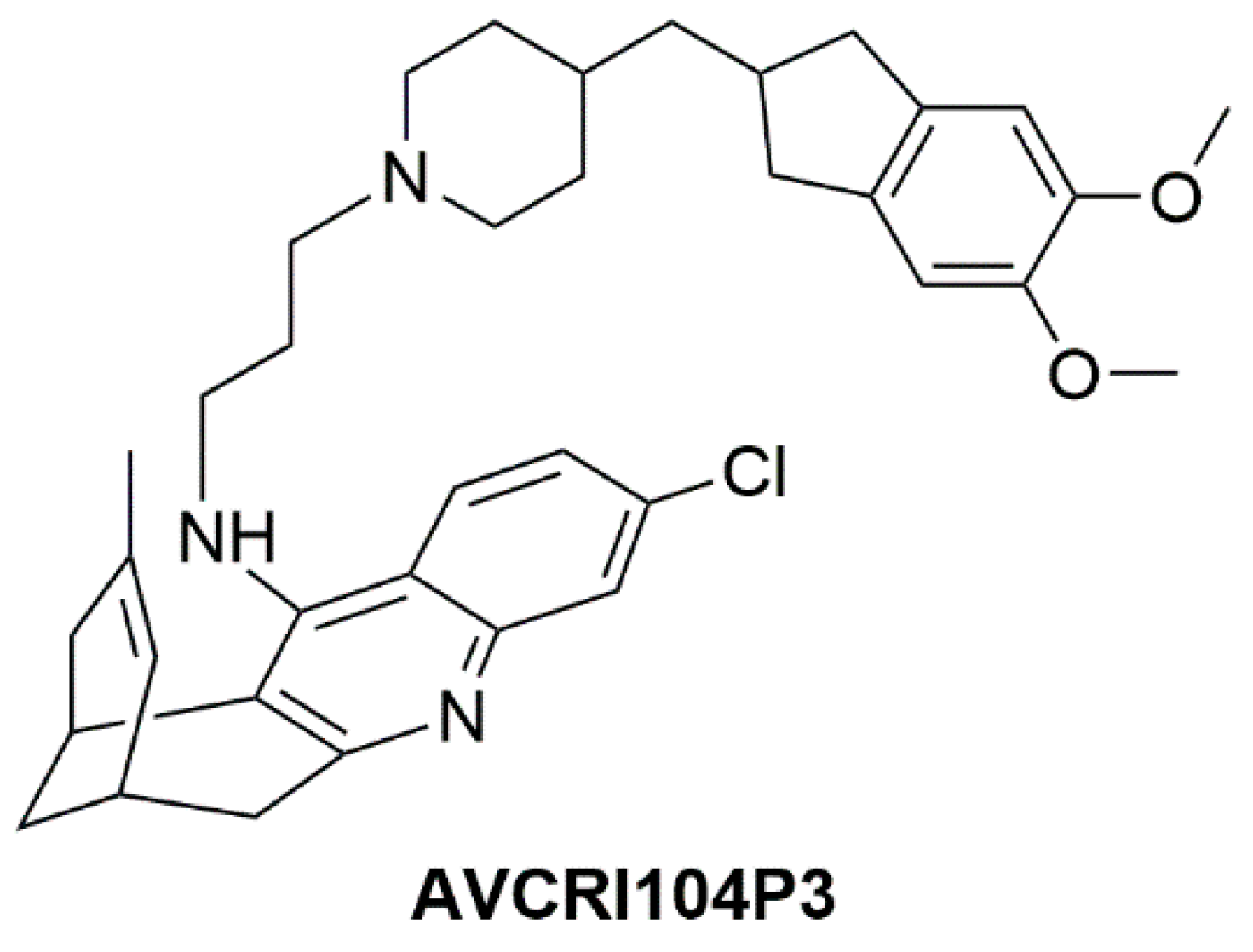
- Giménez-Llort, L.; Ratia, M.; Pérez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Clos, M.V. AVCRI104P3, a novel multitarget compound with cognition-enhancing and anxiolytic activities: Studies in cognitively poor middle-aged mice. Behav. Brain Res. 2015, 286, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, G.; Njunting, M.; Ivens, S.; Tolner, E.A.; Behrens, C.J.; Gross, M.; Soreq, H.; Heinemann, U.; Friedman, A. Acetylcholine-induced seizure-like activity and modified cholinergic gene expression in chronically epileptic rats. Eur. J. Neurosci. 2008, 27, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Darreh-Shori, T.; Kadir, A.; Almkvist, O.; Grut, M.; Wall, A.; Blomquist, G.; Eriksson, B.; Långström, B.; Nordberg, A. Inhibition of acetylcholinesterase in CSF versus brain assessed by 11C-PMP PET in AD patients treated with galantamine. Neurobiol. Aging 2008, 29, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.L.; Hague, A. The PI3K/Akt pathway in tumors of endocrine tissues. Front. Endocrinol. 2016, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Janeczek, M.; Gefen, T.; Samimi, M.; Kim, G.; Weintraub, S.; Bigio, E.; Rogalski, E.; Mesulam, M.-M.; Geula, C. Variations in acetylcholinesterase activity within human cortical pyramidal neurons across age and cognitive trajectories. Cereb. Cortex 2018, 28, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Dikshit, M.; Nath, C. Profile of acetylcholinesterase in brain areas of male and female rats of adult and old age. Life Sci. 2001, 68, 1545–1555. [Google Scholar] [CrossRef]
- Floyd, R.; Hensley, K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Navarro, A.; Boveris, A. Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv. Drug Deliv. Rev. 2008, 60, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Badia, A.; Camps, P.; Clos, M.V. Potentiation effects of (+/−)huprine X, a new acetylcholinesterase inhibitor, on nicotinic receptors in rat cortical synaptosomes. Neuropharmacology 2004, 46, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Yonezawa, A.; Kume, T.; Katsuki, H.; Kaneko, S.; Sugimoto, H.; Akaike, A. Nicotinic acetylcholine receptor-mediated neuroprotection by donepezil against glutamate neurotoxicity in rat cortical neurons. J. Pharmacol. Exp. Ther. 2003, 306, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Kihara, T.; Shimohama, S.; Sawada, H.; Honda, K.; Nakamizo, T.; Shibasaki, H.; Kume, T.; Akaike, A. Alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J. Biol. Chem. 2001, 276, 13541–13546. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhou, M.; Lü, C.; Hu, X.; Wan, B.; Yang, B.; Li, Y. Effects of triptolide on the synaptophysin expression of hippocampal neurons in the AD cellular model. Int. Immunopharmacol. 2012, 13, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tian, J.; Zhang, X.; Wei, M.; Yin, L.; Wang, P.; Wang, Y. A combination extract of ginseng, epimedium, polygala, and tuber curcumae increases synaptophysin expression in APPV717I transgenic mice. Chin. Med. 2012, 7, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordá, E.G.; Verdaguer, E.; Jiménez, A.; Canudas, A.M.; Rimbau, V.; Camps, P.; Muñoz-Torrero, D.; Camins, A.; Pallàs, M. (+/−)-huprine Y, (−)-huperzine A and tacrine do not show neuroprotective properties in an apoptotic model of neuronal cytoskeletal alteration. J. Alzheimers Dis. 2004, 6, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ki, Y.S.; Park, E.Y.; Lee, H.W.; Oh, M.S.; Cho, Y.W.; Kwon, Y.K.; Moon, J.H.; Lee, K.T. Donepezil, a potent acetylcholinesterase inhibitor, induces caspase-dependent apoptosis in human promyelocytic leukemia HL-60 cells. Biol. Pharm. Bull. 2010, 33, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Q.; Zhang, H.Y.; Tang, X.C. Huperzine A attenuates amyloid beta-peptide fragment 25-35-induced apoptosis in rat cortical neurons via inhibiting reactive oxygen species formation and caspase-3 activation. J. Neurosci. Res. 2002, 67, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, K.; Yan, M.; Wang, Y.; Zheng, X. Protective effects of galantamine against Aβ-induced PC12 cell apoptosis by preventing mitochondrial dysfunction and endoplasmic reticulum stress. Neurochem. Int. 2010, 57, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kihara, T.; Hongo, H.; Wu, X.; Kem, W.R.; Shimohama, S.; Akaike, A.; Niidome, T.; Sugimoto, H. Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of alpha7 nicotinic receptors and internalization of NMDA receptors. Br. J. Pharmacol. 2010, 161, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Means, J.C.; Gerdes, B.C.; Kaja, S.; Sumien, N.; Payne, A.J.; Stark, D.A.; Borden, P.K.; Price, J.L.; Koulen, P. Caspase-3-dependent proteolytic cleavage of tau causes neurofibrillary tangles and results in cognitive impairment during normal aging. Neurochem. Res. 2016, 41, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.; Phaneuf, S.; Dirks, A.; Leeuwenburgh, C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann. N. Y. Acad. Sci. 2002, 959, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Godbout, J.P.; Johnson, R.W. Age and neuroinflammation: A lifetime of psychoneuroimmune consequences. Immunol. Allergy Clin. N. Am. 2009, 29, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.W.; Claus, R.A.; Witte, O.W.; Frahm, C. Attenuated inflammatory response in aged mice brains following stroke. PLoS ONE 2011, 6, e26288. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Hattiangady, B.; Shetty, A.K. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell 2006, 5, 545–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makitani, K.; Nakagawa, S.; Izumi, Y.; Akaike, A.; Kume, T. Inhibitory effect of donepezil on bradykinin-induced increase in the intracellular calcium concentration in cultured cortical astrocytes. J. Pharmacol. Sci. 2017, 134, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Consolim-Colombo, F.M.; Sangaleti, C.T.; Costa, F.O.; Morais, T.L.; Lopes, H.F.; Motta, J.M.; Irigoyen, M.C.; Bortoloto, L.A.; Rochitte, C.E.; Harris, Y.T.; et al. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight 2017, 2, 93340. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gurun, M.S.; Flood, P.; Papke, R.L.; Damaj, M.I. New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: A focus on α7 nAChRs. Curr. Neuropharmacol. 2018, 16, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.A.; Kulshrestha, M.; Rogers, D.T.; Littleton, J.M. A nicotinic receptor-mediated anti-inflammatory effect of the flavonoid rhamnetin in BV2 microglia. Fitoterapia 2014, 98, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shytle, R.D.; Mori, T.; Townsend, K.; Vendrame, M.; Sun, N.; Zeng, J.; Ehrhart, J.; Silver, A.A.; Sanberg, P.R.; Tan, J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J. Neurochem. 2004, 89, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K.; Xie, L.; Mao, X.O.; Greenberg, D.A. Alzheimer’s disease drugs promote neurogenesis. Brain Res. 2006, 1085, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Campanari, M.L.; Navarrete, F.; Ginsberg, S.D.; Manzanares, J.; Sáez-Valero, J.; García-Ayllón, M.S. Increased expression of readthrough acetylcholinesterase variants in the brains of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2016, 53, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Darreh-Shori, T.; Soininen, H. Effects of cholinesterase inhibitors on the activities and protein levels of cholinesterases in the cerebrospinal fluid of patients with Alzheimer’s disease: A review of recent clinical studies. Curr. Alzheimer Res. 2010, 7, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livneh, U.; Dori, A.; Katzav, A.; Kofman, O. Strain and regional dependence of alternate splicing of acetylcholinesterase in the murine brain following stress or treatment with diisopropylfluorophosphate. Behav. Brain Res. 2010, 210, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Moral-Naranjo, M.T.; Montenegro, M.F.; Munoz-Delgado, E.; Campoy, F.J.; Vidal, C.J. The levels of both lipid rafts and raft-located acetylcholinesterase dimers increase in muscle of mice with muscular dystrophy by merosin deficiency. Biochim. Biophys. Acta 2010, 1802, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Relat, J.; Come, J.; Perez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Gimenez-Llort, L.; Clos, M.V. Neuroprotective Effects of the Multitarget Agent AVCRI104P3 in Brain of Middle-Aged Mice. Int. J. Mol. Sci. 2018, 19, 2615. https://doi.org/10.3390/ijms19092615
Relat J, Come J, Perez B, Camps P, Muñoz-Torrero D, Badia A, Gimenez-Llort L, Clos MV. Neuroprotective Effects of the Multitarget Agent AVCRI104P3 in Brain of Middle-Aged Mice. International Journal of Molecular Sciences. 2018; 19(9):2615. https://doi.org/10.3390/ijms19092615
Chicago/Turabian StyleRelat, Julia, Julio Come, Belen Perez, Pelayo Camps, Diego Muñoz-Torrero, Albert Badia, Lydia Gimenez-Llort, and M. Victòria Clos. 2018. "Neuroprotective Effects of the Multitarget Agent AVCRI104P3 in Brain of Middle-Aged Mice" International Journal of Molecular Sciences 19, no. 9: 2615. https://doi.org/10.3390/ijms19092615